In a 180-day, randomized, double-blind trial, Rivfloza® was compared with placebo in patients aged 6 years or older with primary hyperoxaluria type 1 (PH1) or primary hyperoxaluria type 2 (PH2). Patients received monthly doses of Rivfloza® (n=23) or placebo (n=12).1
Too few patients with PH2 were enrolled to evaluate efficacy in the PH2 population. Therefore, Rivfloza® is only indicated in patients with PH1.1
The efficacy of Rivfloza® was evaluated in the PHYOXTM2 pivotal study1
PHYOX™2 was a randomized, double-blind, placebo-controlled study of 35 patients aged ≥6 years with PH1 (n=29) or PH2 (n=6) and eGFR ≥30 mL/min/1.73m2.1
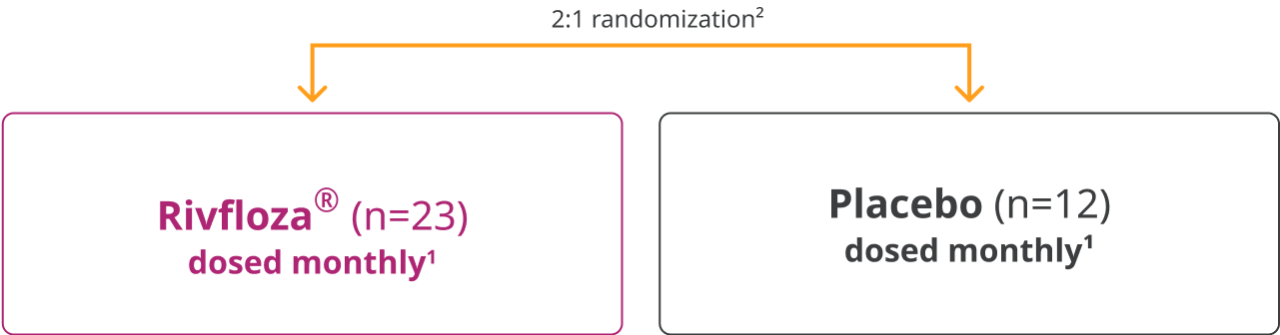
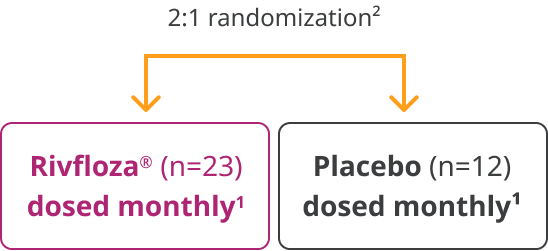
Primary endpoint1:
Percent change from baseline in 24-hour UOxa excretion from Day 90 to Day 180 (LS mean assessed by area under the curve 24-hour UOx [AUC24-hour UOx]).
Baseline characteristics were as follows:
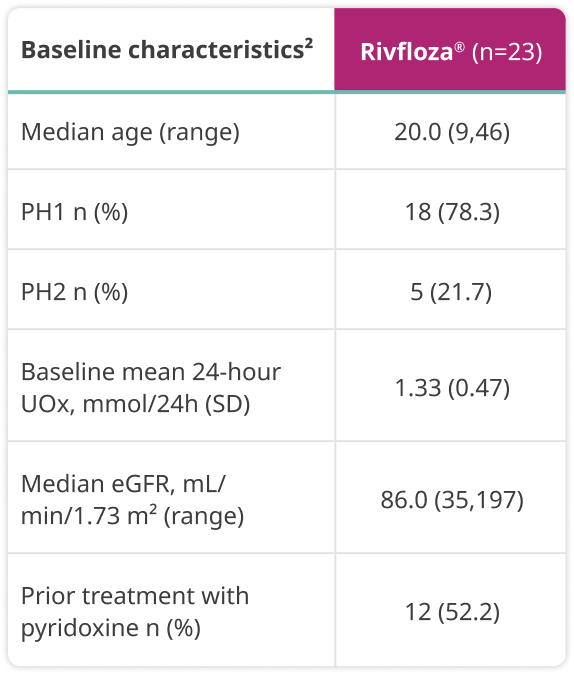
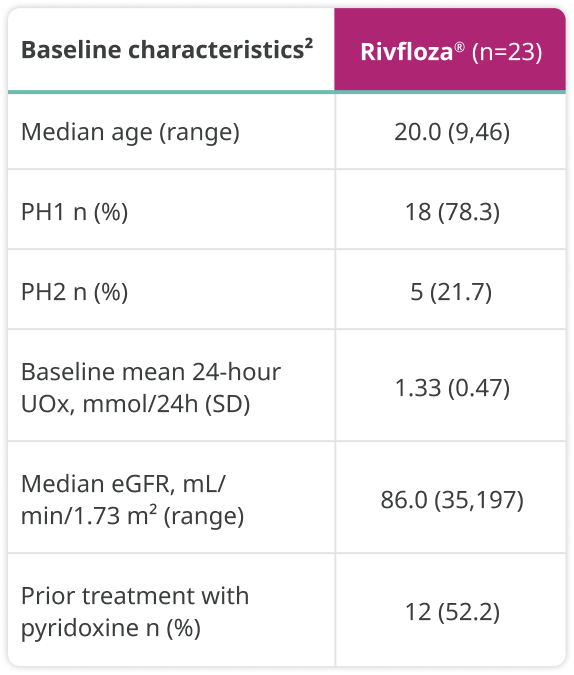
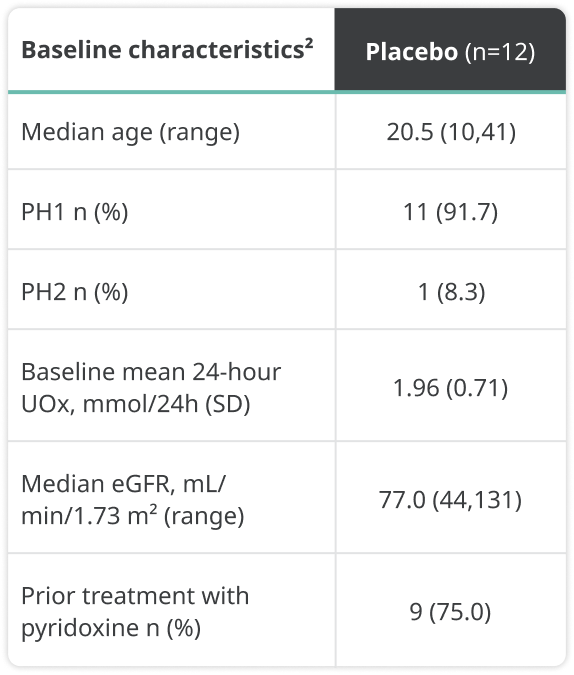
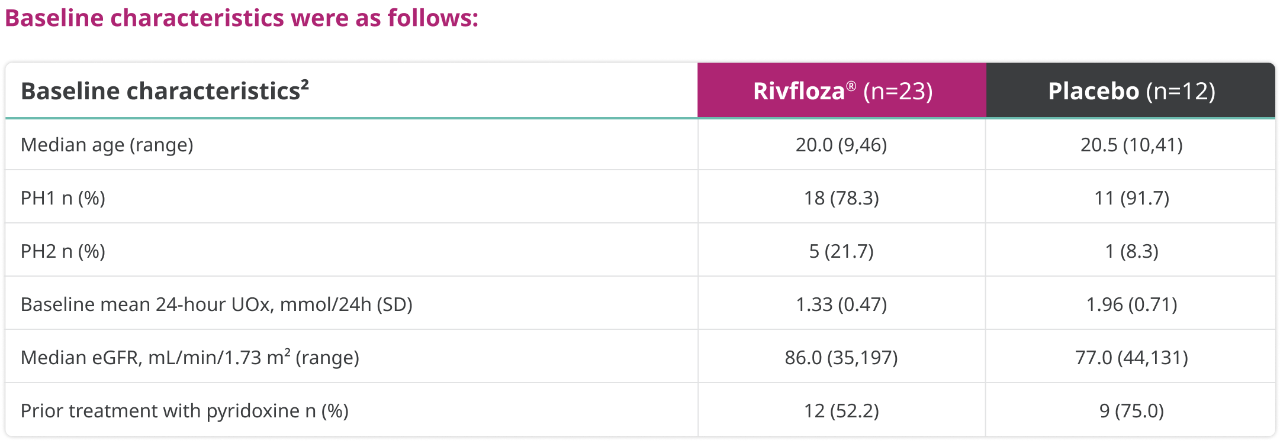
a24-hour UOx values were adjusted by 1.73 m2 BSA in patients aged <18 years.1
BSA=body surface area; eGFR=estimated glomerular filtration rate; LS=least squares; SD=standard deviation.
Efficacy results from PHYOXTM2
Primary endpoint1: percent change from baseline in 24-hour UOxa excretion from Day 90 to Day 180 (LS mean assessed by area under the curve 24-hour UOx [AUC24-hour UOx]).
In patients with PH1 or PH2, the LS mean AUC24-hour UOx in the Rivfloza® arm (n=23) was -3486 vs 1490 in the placebo arm (n=12), resulting in an LS mean difference of 4976 (95% CI: 2803, 7149; P<0.0001).
Change from baseline in 24-hour UOx in patients with PH13
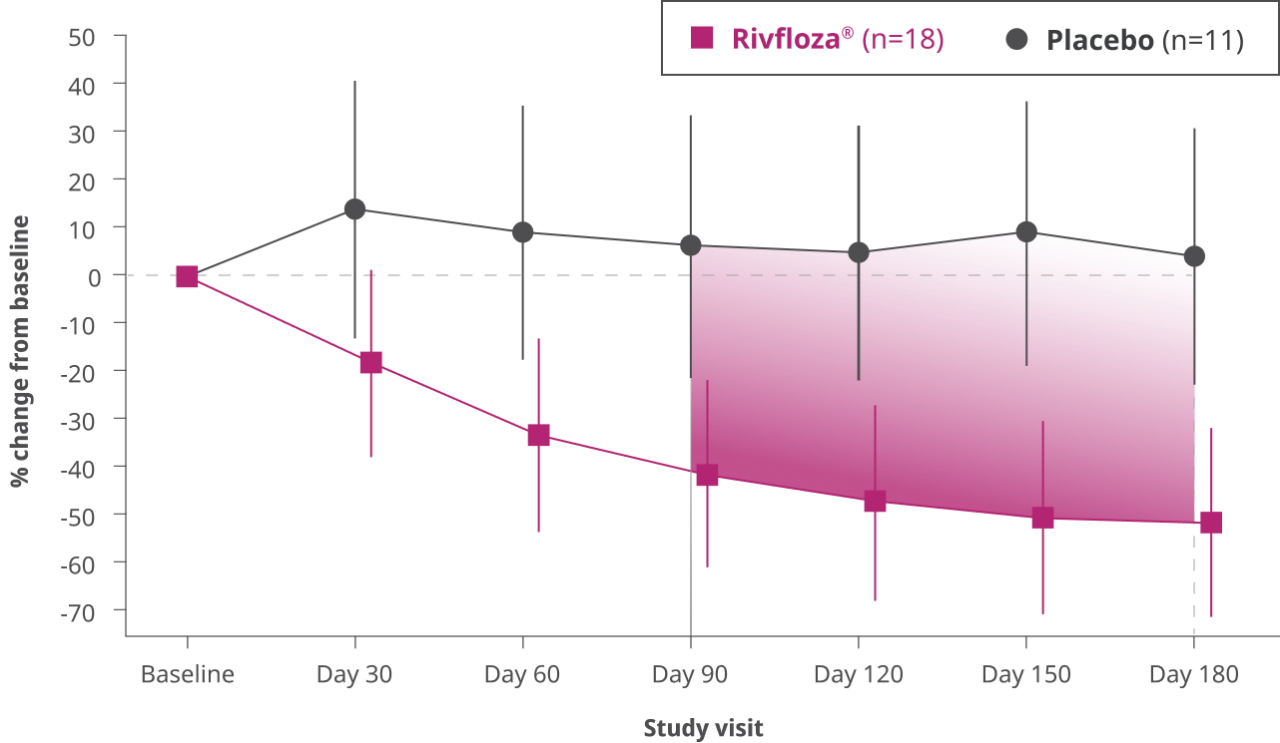
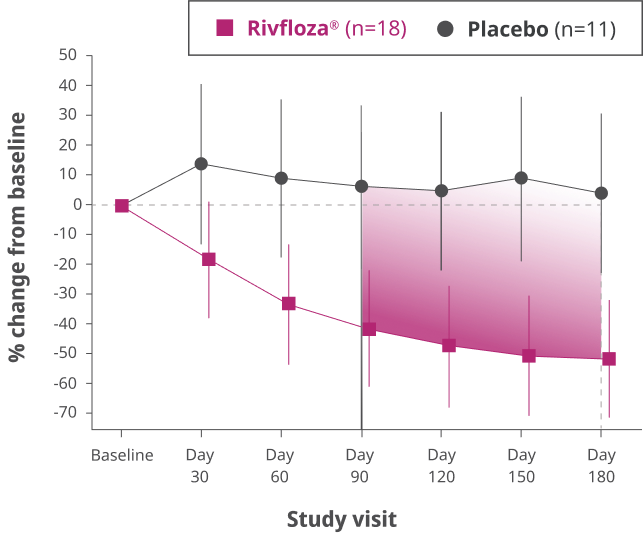
Difference in LS mean percent change in UOx from baseline compared to placebo, measured monthly from Day 90 to Day 180 in patients with PH1 (95% CI: 33%, 80%).3
The reduction in UOx was maintained in all 13 patients with PH1 who received an additional 6 months of treatment with Rivfloza® in PHYOX™3, an ongoing single-arm extension study.1
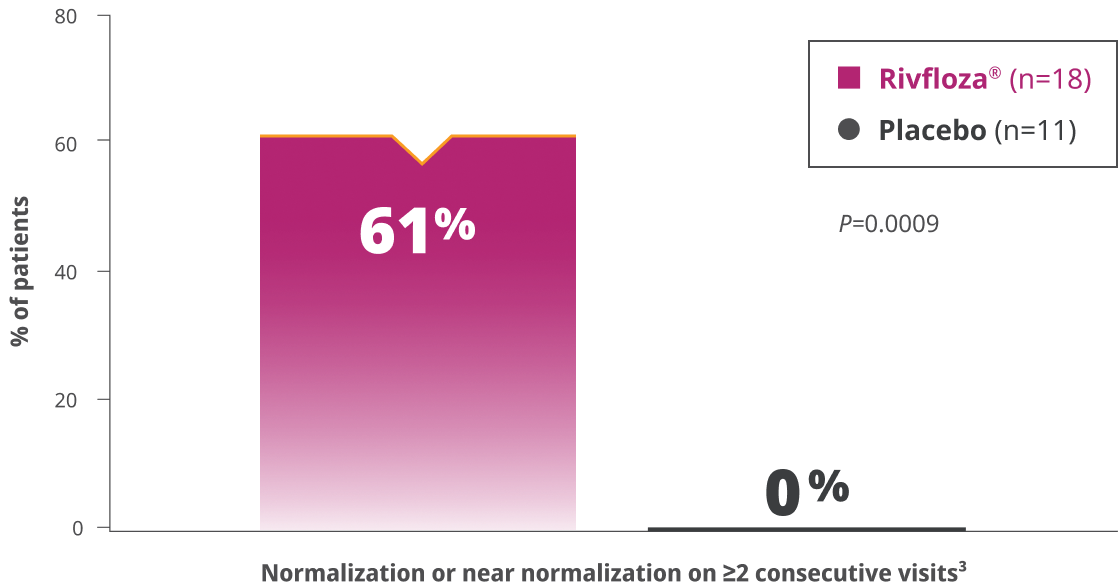
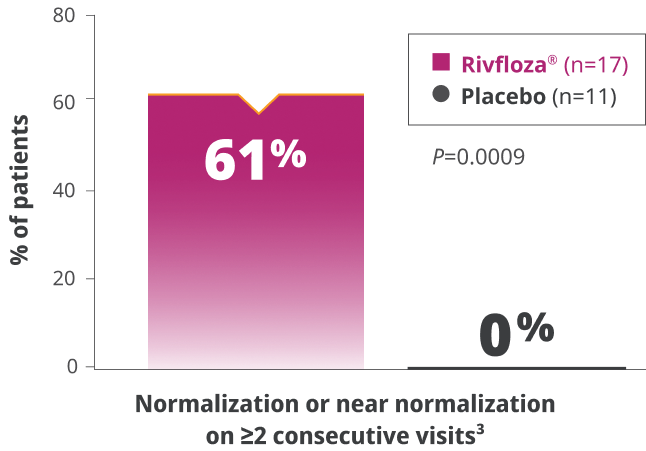
of PH1 patients treated with Rivfloza® (n=17)a had normal or near-normal 24-hour UOx at Day 180.3
aPercentage is based on the number of participants in the ITT PH1 population with 24-hour UOx meeting completeness criteria.3
ITT=intent to treat.
Additional endpoints: stone assessment in patients with PH1 treated with Rivfloza®
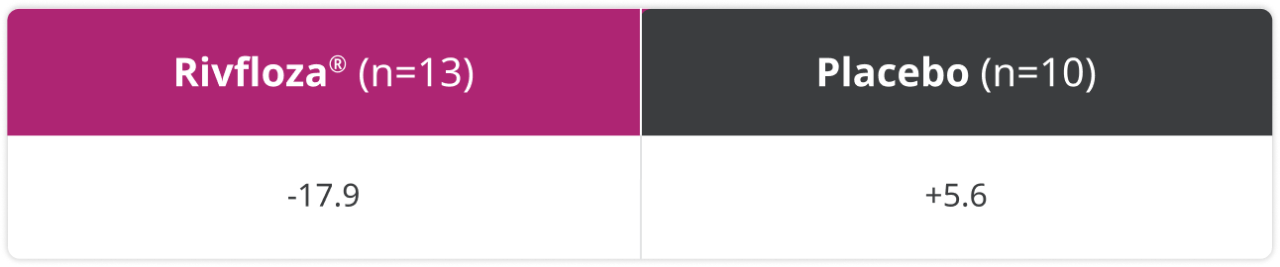
Prespecified secondary endpoint: In a post-hoc analysis, summed kidney stone surface area was measured at Day 180 via kidney ultrasound.2
Median percent change in summed kidney stone surface area (mm2) from baseline to Day 180 in patients with PH12

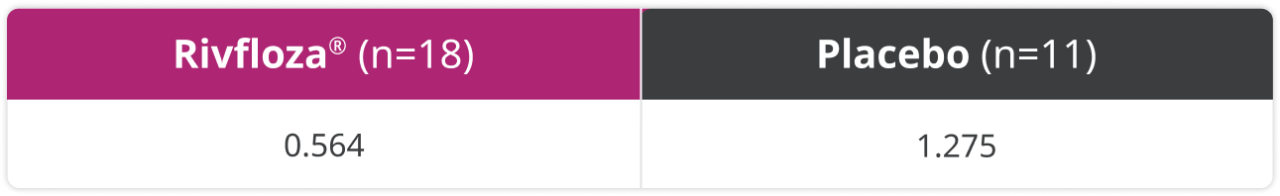
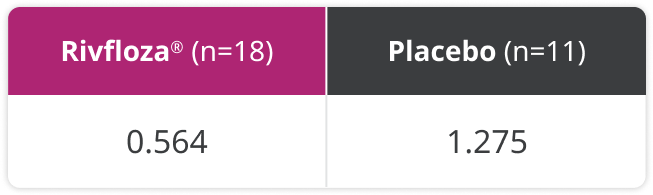
Exploratory endpoint: In a post-hoc analysis, the annualized stone event rate was studied.3
Annualized kidney stone event rate in patients with PH13
Limitations: These subanalyses were included in the study as exploratory endpoints and not adjusted for multiplicity or powered to determine statistical significance.
There was no prespecified statistical analysis for controlling for false positive rate when conducting multiple analyses, and alpha was not allocated. No efficacy conclusions can be drawn from these analyses.
Rivfloza® was safe and well-tolerated
No serious treatment-related adverse events in patients with PH1 who received Rivfloza® in the pivotal trial1
Most common adverse reactions reported in ≥20% of patients with PH1 studied in PHYOX™2.1


- Injection site reactions included erythema, pain, bruising, and rash and were generally mild and did not lead to discontinuation of treatment.1
- Across all clinical studies, Rivfloza® did not induce or boost ADAs. Among 59 patients tested with an ADA assay, none developed treatment-emergent ADAs.1
ADA=antidrug antibody.

ADA=antidrug antibody.
Important Safety Information for Rivfloza®
Adverse Reactions
- Most common adverse reaction (reported in 39% of patients) are injection site reactions, which include erythema, pain, bruising, and rash.
Indications and Usage
Rivfloza® (nedosiran) injection 80 mg, 128 mg, or 160 mg is indicated to lower urinary oxalate levels in children 9 years of age and older and adults with primary hyperoxaluria type 1 (PH1) and relatively preserved kidney function, eg, eGFR ≥30 mL/min/1.73 m2.
Please click here for Rivfloza® Prescribing Information.
Important Safety Information for Rivfloza®
Adverse Reactions
- Most common adverse reaction (reported in 39% of patients) are injection site reactions, which include erythema, pain, bruising, and rash.
Indications and Usage
Rivfloza® (nedosiran) injection 80 mg, 128 mg, or 160 mg is indicated to lower urinary oxalate levels in children 9 years of age and older and adults with primary hyperoxaluria type 1 (PH1) and relatively preserved kidney function, eg, eGFR ≥30 mL/min/1.73 m2.
Please click here for Rivfloza® Prescribing Information.
References:
- Rivfloza® [package insert]. Plainsboro, NJ: Novo Nordisk Inc.
- Baum MA, Langman C, Cochat P, et al. PHYOX2 study investigators. PHYOX2: a pivotal randomized study of nedosiran in primary hyperoxaluria type 1 or 2. Kidney Int. 2023;103(1):207-217. doi:10.1016/j.kint.2022.07.025
- Data on file. Novo Nordisk Inc; Plainsboro, NJ.
