At-home self-administration1
At-home self-administration1
After proper training:
- Rivfloza® can be self- or caregiver-administered at home using a single-dose prefilled syringe or by vial.
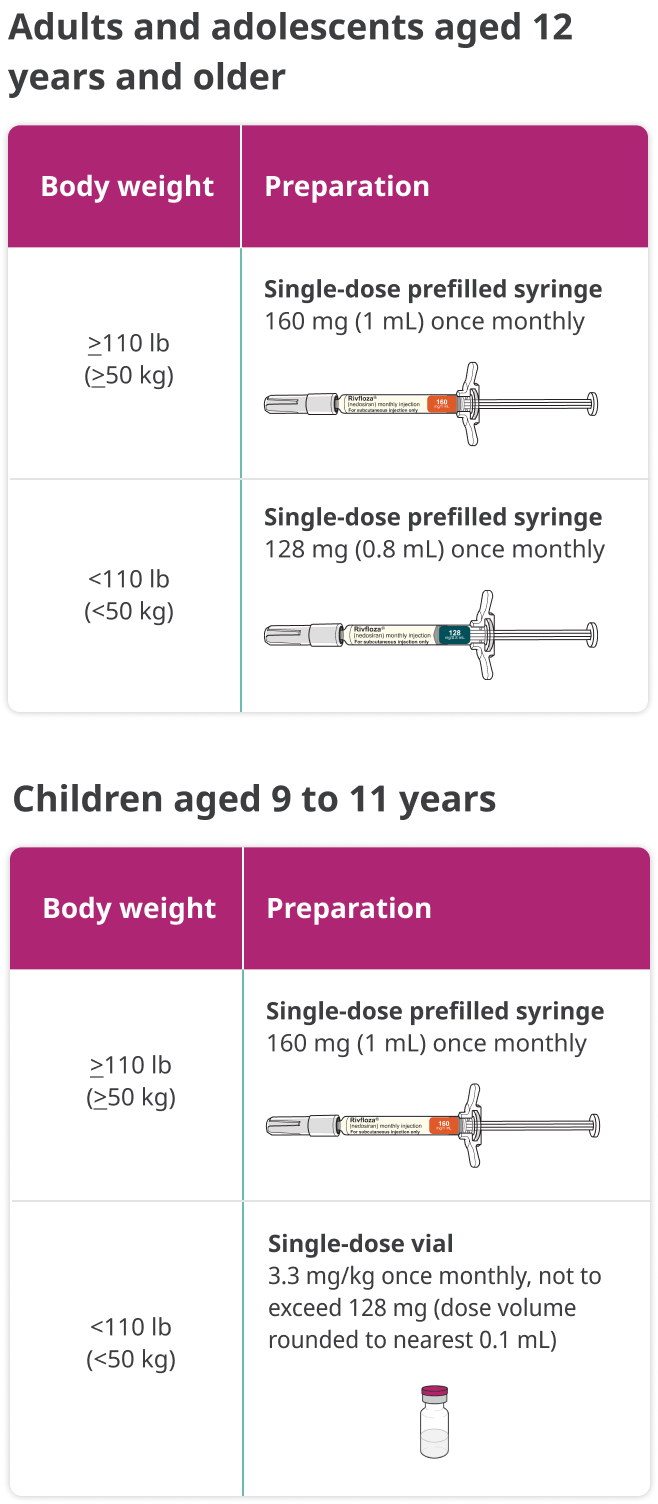
- For children aged 9 to 11 years, Rivfloza® may be administered by a caregiver under guidance and supervision of a health care professional.

Monthly dosing1
Monthly dosing1
- Rivfloza® is administered once monthly via subcutaneous injection at the recommended weight- and age-based dose.
- No loading dose or titration schedule is required.
- If a patient misses a dose, advise to inject the dose as soon as possible. If a dose is missed by more than 7 days, inject the dose as soon as possible and resume monthly dosing from the most recently injected dose.


Dosing regimen for Rivfloza® is determined by age and body weight1
Discover which dose of Rivfloza® you may want to prescribe for your patients with PH1.

Learn more about administering Rivfloza®
For patients using the prefilled syringe

This is a demonstration of how to prepare and administer Rivfloza®. Read the Instructions for Use provided with the medication before using Rivfloza® and each time you refill. There may be new information. Please see the accompanying Prescribing Information (PI), including Instructions for Use, located in each carton of Rivfloza®. Ask your or your child’s healthcare provider if you have any questions. To learn more about Rivfloza® and available support programs, visit Rivfloza.com.
What is Rivfloza®? Rivfloza® (nedosiran) injection 80 mg, 128 mg, or 160 mg is a prescription medicine used to lower urinary oxalate levels in children 9 years of age and older and adults with primary hyperoxaluria type 1 (PH1) and relatively preserved kidney function. It is not known if Rivfloza® is safe and effective in children younger than 9 years of age.
Important Safety Information. What are the possible side effects of Rivfloza®? The most common side effects of Rivfloza® include injection site reactions, such as reddening, pain, bruising, rash, or dimple at the site of injection.
Important information you need to know before injecting Rivfloza®. Your healthcare provider will show you how to prepare and inject Rivfloza® before you use the Pre-filled Syringe for the first time. Use Rivfloza® Pre-filled Syringe exactly as your healthcare provider tells you to. In children 9 to 11 years of age weighing 110 pounds (50 kilograms) or more, it is recommended that Rivfloza® Pre-filled Syringe be given by a healthcare provider or caregiver. Your healthcare provider will tell you how much Rivfloza® to inject and when to inject it.
Rivfloza® Pre-filled Syringe is a single-dose Pre-filled Syringe for one-time (single) use only. Do not reuse your Pre-filled Syringe. Rivfloza® Pre-filled Syringe is for injection under the skin (subcutaneous injection) only. Do not inject Rivfloza® into a vein. Your Rivfloza® injection mat is available in the Rivfloza® starter kit or by calling NovoCare® at 1-844-906-5099.
Preparing Your Injection. Remove the Rivfloza® Pre-filled Syringe carton from the refrigerator. Make sure the carton contains the correct dose. Check expiration date. Wait 30 minutes to allow the medicine to reach room temperature. Caution: Do not warm the Pre-filled Syringe using any heat sources such as hot water or a microwave. Wash your hands with soap and water. Grip the barrel of the Pre-filled Syringe and remove it from the carton. Inspect the Pre-filled Syringe. Look at the medicine inside. It should appear colorless-to-yellow and free of particles. Gather supplies and place them on a clean, flat surface in a well-lit area. The Rivfloza® injection mat works well to help you gather your supplies.
Choose your injection site. Medicine may be injected into the skin of the stomach area at least 2 inches from your belly button, or on the upper thigh. Clean the injection site with an alcohol wipe and let it air dry. Caution: Do not inject into scarred or bruised skin.
Giving Your Injection. Remove the needle cap and throw it away in the sharps disposal container. Hold the Pre-filled Syringe with one hand with needle pointed away from you. Pull the needle cap straight off with the other hand. Caution: If the needle appears to be bent or damaged, do not use the Pre-filled Syringe. Caution: Do not touch or recap the needle. Caution: Do not touch the plunger until you are ready to inject. Pinch skin around the injection site with one hand. Grasp the finger grip portion of the Pre-filled Syringe with the other hand, and fully insert the needle into the injection site at a 45-degree angle. Slowly inject all the medicine. Gently push the plunger rod all the way down until the Pre-filled Syringe is empty. The rubber stopper inside the Pre-filled Syringe moves to the bottom of the barrel as medicine is injected. Remove the Pre-filled Syringe from the injection site. Caution: Do not recap the needle. If there is bleeding, lightly press a cotton ball or gauze over the injection site.
After Your Injection. Throw away or dispose of the used Pre-filled Syringe. Put the used Pre-filled Syringe in the FDA-cleared sharps disposal container right away after use. If an FDA-cleared sharps disposal container is not available, you may use a household container that is: Made of heavy duty plastic, can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out, upright and stable during use, leak-resistant, and properly labeled to warn of hazardous waste inside the container. You should throw away unused portion of medicine. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at www.fda.gov/safesharpsdisposal.
Please see the Prescribing Information (PI), including Instructions for Use, located in each carton of Rivfloza®. NovoCare® is here to assist you if you have any questions Monday-Friday 8 am – 8 pm EST, at 1-844-906-5099.
US23RVZA00068
For patients using the single-dose vial

This is a demonstration of how to prepare and administer Rivfloza®. Read the Instructions for Use provided with the medication before using Rivfloza® and each time you refill. There may be new information. Please see the accompanying Prescribing Information (PI), including Instructions for Use, located in each carton of Rivfloza®. Ask your or your child’s healthcare provider if you have any questions. To learn more about Rivfloza® and available support programs, visit Rivfloza.com. Ask your or your child’s healthcare provider if you have any questions. To learn more about Rivfloza® and available support programs, visit Rivfloza.com.
What is Rivfloza®? Rivfloza® (nedosiran) injection 80 mg, 128 mg, or 160 mg is a prescription medicine used to lower urinary oxalate levels in children 9 years of age and older and adults with primary hyperoxaluria type 1 (PH1) and relatively preserved kidney function. It is not known if Rivfloza® is safe and effective in children younger than 9 years of age.
Important Safety Information. What are the possible side effects of Rivfloza®? The most common side effects of Rivfloza® include injection site reactions, such as reddening, pain, bruising, rash, or dimple at the site of injection. Important information you need to know before injecting Rivfloza®. Your child’s healthcare provider will show you how to prepare and inject Rivfloza®. Do not try to inject Rivfloza® until you have been shown the right way by your child’s healthcare provider. Use Rivfloza® vials exactly as your child’s healthcare provider tells you to. Your child’s healthcare provider will tell you how much Rivfloza® to inject and when to inject it. Rivfloza® vials are for one-time use (single-dose) only. Do not reuse the Rivfloza® vial. Throw away (or discard of) any unused Rivfloza®. Rivfloza® is for injection under the skin (subcutaneous injection) only. Do not inject Rivfloza® into a vein. Your Rivfloza® injection mat is available in the Rivfloza® starter kit or by calling NovoCare® at 1-844-906-5099.
Preparing Your Injection. Remove the Rivfloza® vial carton(s) from the refrigerator. Check the expiration date on the carton or cartons. Wait 30 minutes to allow the medication to reach room temperature. Caution: Do not warm the vial using any heat sources such as hot water or a microwave. Your medication may have 1 or 2 vials of Rivfloza® and 1 or 2 syringes depending on the prescribed dose. Wash your hands with soap and water. Open the carton or cartons and remove the vial or vials. Check the vial labels to make sure it’s the correct medicine for your child’s prescription. Look at the medicine in the vial. It should appear colorless-to-yellow and free of particles. Gather supplies and place them on a clean, flat surface in a well-lit area. The Rivfloza® injection mat works well to help you gather your supplies.
Choose your injection site. Medicine may be injected into the skin of the stomach area at least 2 inches from the belly button, or on the upper thigh. Clean the injection site or sites with alcohol wipe and let air dry. Caution: Do not inject into scarred or bruised skin. Do not inject the contents of 2 syringes into the same location.
Prepare your vial or vials. Remove the cap from the vial, clean the top of the gray rubber stopper with a new alcohol wipe.
Giving Your Injection. Your provided syringe may look slightly different. Remove the syringe with the attached needle from the packaging and remove the needle cap. Throw the needle cap away in the sharps disposal container. Caution: Take care when handling the uncapped needle. Do not touch the uncapped needle. If your child’s dose is 0.5 mL or less insert the needle into the gray rubber stopper on top of the vial. Turn the vial and syringe upside down. Keep the tip of needle in the medicine. Hold syringe and vial in one hand. With the other hand, slowly pull back on the plunger rod to withdraw child’s prescribed dose into the syringe. If your child’s dose is 0.6 mL or more: You will need to withdraw your child’s dose of Rivfloza® from 2 vials using 2 separate syringes. Follow “If your child’s dose is 0.5 mL or less” instructions to withdraw the amount of medicine needed from each vial.
Follow these steps for each syringe. If large air bubbles appear in the syringe, tap the side of the syringe to move the air bubbles to the top of the syringe. Push the plunger rod up to push the air bubbles back into the vial. If the syringe doesn’t contain the correct dose after the air bubbles have been removed, pull back on the plunger rod again to the prescribed dose. Turn the vial and syringe back upright and remove the needle from the vial. Look at the syringe to make sure you have the correct amount for your child’s dose and it is free of large air bubbles. Pinch the skin around the injection site with one hand. With the other hand, fully insert the needle into the skin at a 45-degree angle. Slowly inject all the medicine. Gently push the plunger rod all the way down until the syringe is empty. The rubber stopper inside the syringe moves to the bottom of barrel as medicine is injected. Remove the needle from the injection site. Throw away or dispose of used vial or vials in the household trash.
If there is bleeding, lightly press a cotton ball or gauze over the injection site. If your child’s dose requires 2 vials, follow these steps to inject the second syringe into another area. Remember to inject at least 2 inches away from the belly button and to avoid scarred or bruised skin. Caution: Do not recap the needle. Do not save or keep used syringes.
After Your Injection. Throw away or dispose of the used syringe. Put the used syringe in the FDA-cleared sharps disposal container right away after use. If an FDA-cleared sharps disposal container is not available, you may use a household container that is: Made of heavy duty plastic, can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out, upright and stable during use, leak-resistant, and properly labeled to warn of hazardous waste inside the container. You should throw away unused portion of medicine.
For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at www.fda.gov/safesharpsdisposal.
Please see the Prescribing Information (PI), including Instructions for Use, located in each carton of Rivfloza®. NovoCare® is here to assist you if you have any questions, Monday-Friday 8 am – 8 pm EST, at 1-844-906-5099.
US23RVZA00067
Have questions about Rivfloza®? Reach out to our team of representatives
Important Safety Information for Rivfloza®
Adverse Reactions
- Most common adverse reaction (reported in 39% of patients) are injection site reactions, which include erythema, pain, bruising, and rash.
Indications and Usage
Rivfloza® (nedosiran) injection 80 mg, 128 mg, or 160 mg is indicated to lower urinary oxalate levels in children 9 years of age and older and adults with primary hyperoxaluria type 1 (PH1) and relatively preserved kidney function, eg, eGFR ≥30 mL/min/1.73 m2.
Please click here for Rivfloza® Prescribing Information.
Important Safety Information for Rivfloza®
Adverse Reactions
- Most common adverse reaction (reported in 39% of patients) are injection site reactions, which include erythema, pain, bruising, and rash.
Indications and Usage
Rivfloza® (nedosiran) injection 80 mg, 128 mg, or 160 mg is indicated to lower urinary oxalate levels in children 9 years of age and older and adults with primary hyperoxaluria type 1 (PH1) and relatively preserved kidney function, eg, eGFR ≥30 mL/min/1.73 m2.
Please click here for Rivfloza® Prescribing Information.
Reference:
- Rivfloza® [package insert]. Plainsboro, NJ: Novo Nordisk Inc.
