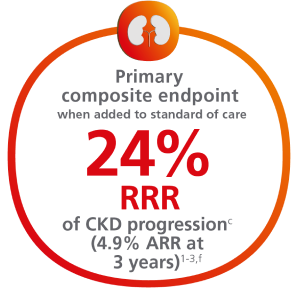
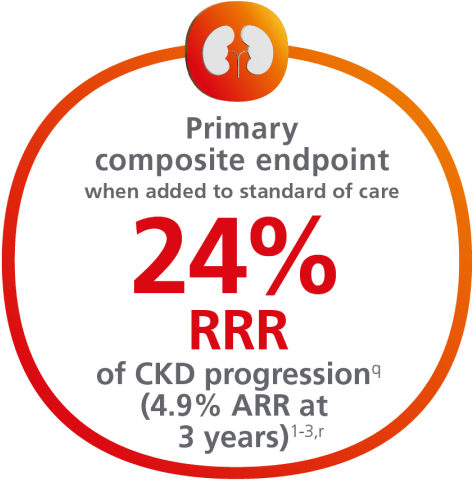
Help protect your patients by reducing their risk of CKD progression1,2
For adults with T2D, for the primary composite endpoint: Time to first occurrence of ≥50% sustained eGFR decline, kidney failure (sustained eGFR <15 mL/min/1.73m2, or initiation of long-term dialysis, or transplantation), or renal or CV death vs placebo when added to standard of care1,2,a
Cumulative incidence: Time to first occurrence of the primary composite endpoint1-3,b,c
aThe eGFR was calculated with serum creatinine level and the Chronic Kidney Disease Epidemiology Collaboration 2009 formula. Events not related to the eGFR were evaluated by the event adjudication committee. Sustained was defined as having 2 consecutive measurements ≥28 days apart by fulfilling the criteria.2
bA cumulative incidence function (CIF) plot graphically shows the probability of an event occurring over time, from randomization to the first event in the presence of competing risks. The relative risk reduction is calculated based on the sum of the events in the trial.4
cCumulative incidence estimates are based on time from randomization to first composite renal event with non-CV and non-renal death modeled as competing risk. The x-axis in truncated at 52 months where approximately 5% of the population was in the trial. Median follow-up was 41 months1,2
dCox proportional hazards model with treatment as factor and stratified by baseline use of SGLT2-inhibitor at baseline (yes or no)
eTwo-side P value for the test of no difference. The Significance level was 0.03224.1
f4.9% ARR reported at 3 years in a prespecified supplementary analysis.3
ARR=absolute risk reduction; CI=confidence interval; CV=cardiovascular; CVD=cardiovascular disease; eGFR=estimated glomerular filtration rate; GLP-1 RA=glucagon-like peptide-1 receptor agonist; RRR=relative risk reduction; SGLT2=sodium-glucose cotransporter 2; T2D=type 2 diabetes.
FLOW evaluated renal and cardiovascular outcomes with Ozempic® once weekly in adults with T2D and CKD
Ozempic® in FLOW: the only GLP-1 RA indicated for T2D and CKD.1,2
A randomized, double-blind, placebo-controlled, event-driven trial that followed 3533 patients with type 2 diabetes and chronic kidney disease (eGFR 25 to 75 mL/min/1.73 m2 with UACR > 100 mg/g and <5000 mg/g) for a median follow-up of 41 months.1,2
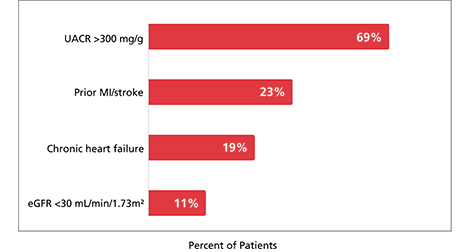
At baseline, patients had a mean eGFR of 47 mL/min/1.73 m2 and a median UACR of 568 mg/g (11% of patients had an eGFR <30 mL/min/1.73 m2 and 69% of patients with UACR >300 mg/g at baseline).1,2
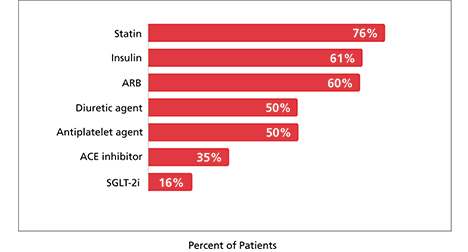
At baseline, 95% of patients were treated with an ACE inhibitor or ARB, 16% were on an SGLT-2i, 76% were on a statin, and 50% were on an antiplatelet agent.1,2
FLOW: kidney outcomes study design
gRandomization was stratified according to SGLT-2 inhibitor use at baseline.2
hAn 8-week dose-escalation regimen was used, with dose escalation from 0.25 mg per week for 4 weeks then 0.5 mg per week for another 4 weeks, followed by maintenance dose of 1 mg per week throughout remainder of the treatment period.2
iDuring the trial, investigators were encouraged to optimize standard-of-care treatment for T2D, CKD, and CVD risk management according to local treatment practice.6
jSustained was defined as having 2 consecutive measurements ≥28 days apart fulfilling the criteria. Death from cardiovascular causes included both death from cardiovascular cause and death from undetermined causes.2
ACE=angiotensin-converting enzyme; ARB=angiotensin II receptor blocker; CI=confidence interval; CKD=chronic kidney disease; CV=cardiovascular; eGFR=estimated glomerular filtration rate; GLP-1 RA=glucagon-like peptide-1 receptor agonist; MACE=major adverse cardiovascular event; OAD=oral antidiabetic drug; RAAS=renin-angiotensin-aldosterone system; SGLT2=sodium glucose co-transporter; T2D=type 2 diabetes; UACR=urine albumin-creatinine ratio.
68% of patients were at very high risk for CKD progression2,k
Patient characteristics at baseline (N=3533)1,2


kPatients were classified as very high risk for CKD progressing according to Kidney Disease: Improving Global Outcomes (KDIGO) risk calculators based on GFR and albuminuria categories.2
lFor eGFR, the baseline assessment is defined as the mean of the two assessments from the randomization visit and the screening visit. If only one of the assessment was available, it was used as the baseline assessment. The mean eGFR is based on the serum creatinine level and the Chronic Kidney Disease Epidemiology Collaboration 2009 equation.2
mThe urinary albumin-to-creatinine ratio was calculated with albumin measured in milligrams and creatinine measured in grams.2
BMI=body mass index; CKD=chronic kidney disease; eGFR=estimated glomerular filtration rate; UACR=urine albumin-creatinine ratio.
Majority of patients were treated with standard of care1,2,n,o
Summary of medication at baseline (N=3533)1,2
Select CV and CKD baseline characteristics (N=3533)1,2
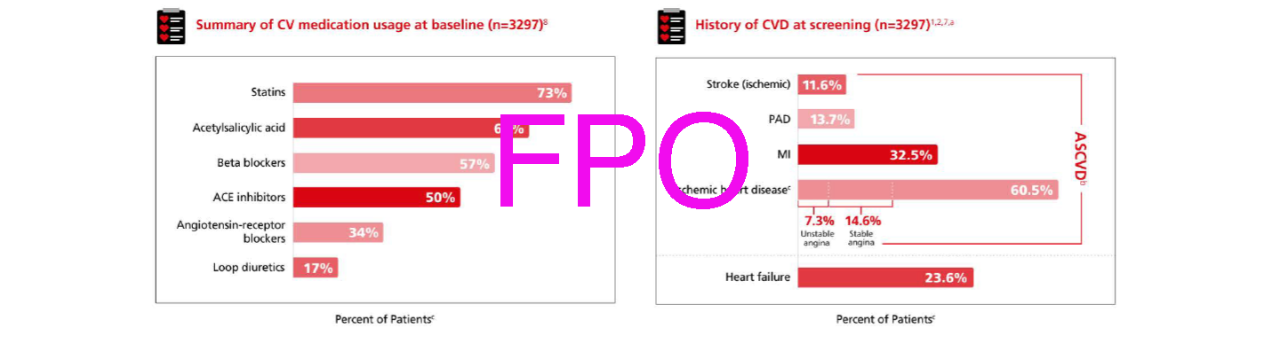
nAt baseline patients in FLOW were receiving standard-of-care background therapy, including a maximum tolerated label dose of a RAAS-blocking agent including an ACE inhibitor or an ARB, unless such treatment was contraindicated or not tolerated.1
oInvestigators were encouraged to optimize standard-of-care treatment for T2D, CKD, and CV risk management according to local treatment practice.6
ACE=angiotensin-converting enzyme; ARB=angiotensin II receptor blocker; CKD=chronic kidney disease; CV=cardiovascular; eGFR=estimated glomerular filtration rate; RAAS=renin-angiotensin-aldosterone system; SLT-2i=sodium glucose co-transporter-2 inhibitor; UACR=urine albumin-creatinine ratio.
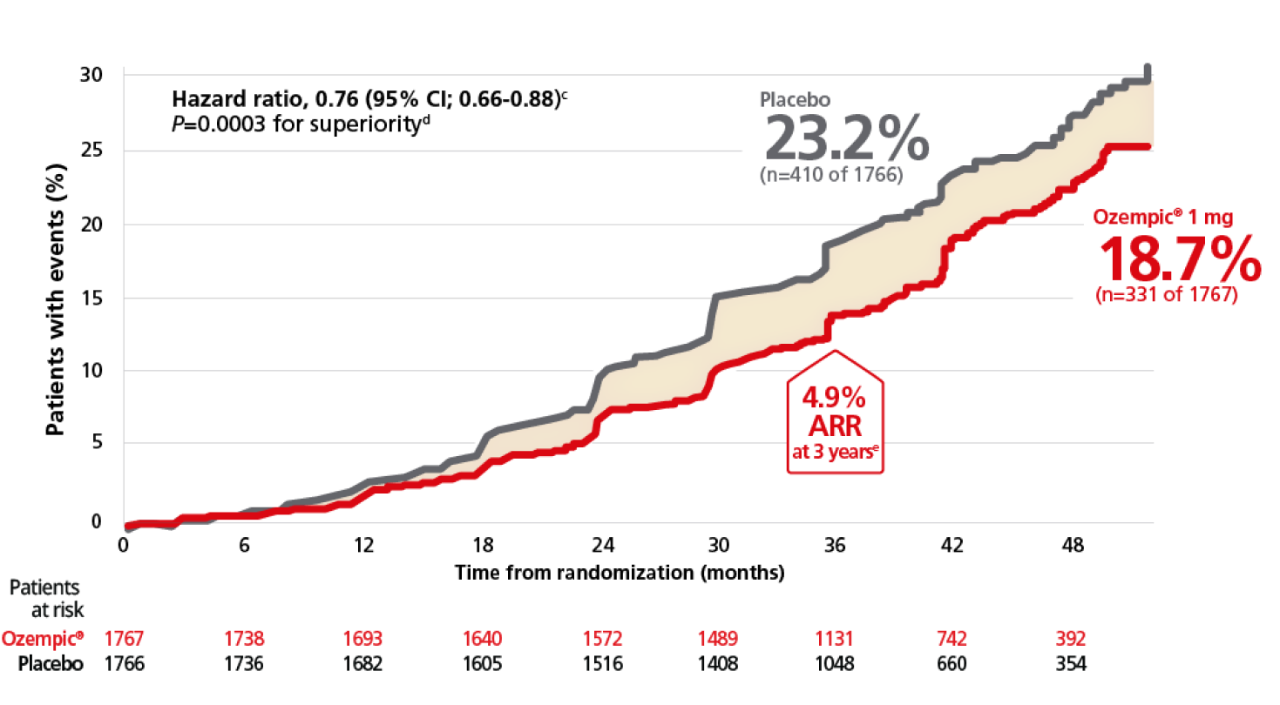
Ozempic® achieved significance in the primary composite endpoint1,2
For adults with T2D and CKD, for the primary composite endpoint: Time to first occurrence of ≥50% sustained eGFR decline, kidney failure (sustained eGFR <15 mL/min/1.73 m2, or initiation of long-term dialysis, or transplantation), or renal or CV death vs placebo when both were added to standard of care1,2,p
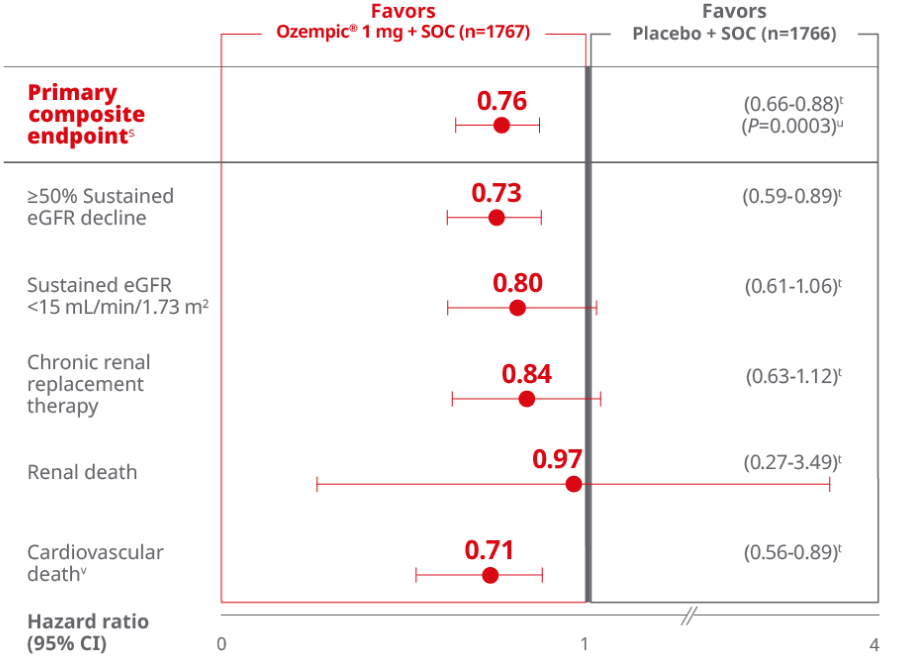
This study was not powered to detect a difference in the individual endpoints of the composite primary endpoint.
Overall, the primary composite endpoint occurred in 331 patients of 1767 (18.7%) on Ozempic® (1 mg) and 410 patients of 1766 (23.2%) on placebo (HR, 0.76 [95% Cl; 0.66-0.88] P=0.0001 for superiority). 1
≥50% sustained eGFR decline occurred in 165 patients (9.3%) on Ozempic® and 213 patients (12.1 %) on placebo. Sustained eGFR <15 mL/min/1.73 m2 occurred in 92 patients (5.2%) on Ozempic® and 110 patients (6.2%} on placebo. Chronic renal replacement therapy occurred in 87 patients (4.9%) on Ozempic® and 100 patients (5.7%) on placebo. Renal death occurred in 5 patients (0.3%} on Ozempic® and 5 patients (0.3%) on placebo. Cardiovascular death occurred in 123 patients (7.0%) on Ozempic® and 169 patients (9.6%) on placebo.1
pSustained was defined as having 2 consecutive measurements ≥28 days apart fulfilling the criteria.1
qA cumulative incidence function (CIF) plot graphically shows the probability of an event occurring over time. From randomization to the first event in the presence of competing risks. The relative risk reduction is calculated based on the sum of events in the trial.4
r4.9% ARR reported at 3 years in prespecified supplementary analysis3
sData are for the full analysis population from the in-trial period (from randomization to the end of trial participation). Data from participants without events of interest were censored at the end of their in-trial period. Median follow-up was 41 months1,2
tCox proportional hazards model with treatment as factor and stratified by baseline use of SGLT2-inhibitor at baseline (yes or no).1
uTwo-sided p value for the test of no difference. The significance level was 0.03224.1
vDeath from cardiovascular causes, as confirmed by the event adjudication committee, includes both death from cardiovascular causes and death from undetermined causes adjudicated by that committee.2
ARR=absolute risk reduction; CI=confidence interval; CKD=chronic kidney disease; CV=cardiovascular; eGFR=estimated glomerular filtration rate; GLP-1 RA=glucagon-like peptide-1 receptor agonist; RRR=relative risk reduction; SOC=standard of care; SLT-2i=sodium glucose co-transporter inhibitor; T2D=type 2 diabetes.
CKD confirmatory secondary endpoints
Significant 18% RRR of MACE with Ozempic®2
For adults with T2D and CKD, for a confirmatory secondary endpoint: Time to first occurrence of MACE (CV death, nonfatal MI, or nonfatal stroke) vs placebo when both were added to standard of care1,2
Time to first major adverse cardiovascular event (MACE)1-3,w-z
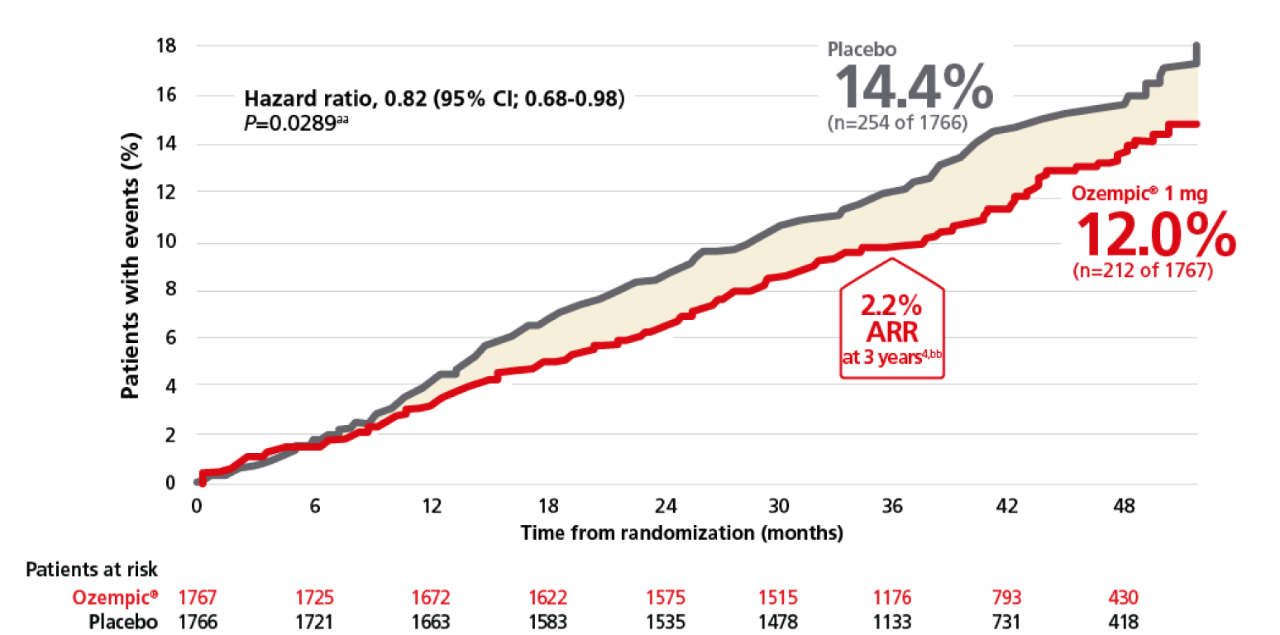
wCumulative incidence estimates are based on time from randomization to first EAC-confirmed MACE with non-CV death modelled as a competing risk using the Aalen-Johnson estimator. The x-axis is truncated at 52 months where approximately 5% of the population was in the trial.1
xComposite major cardiovascular events were analyzed in a time-to-first-event analysis with a Cox proportional-hazards model with treatment as a categorical fixed factor and stratified according to SGLT-2 inhibitor use as baseline.2
yData from participants without events of interest were censored at the end of their in-trial period.2
zDeath from cardiovascular causes, as confirmed by the even adjudication committee, includes both death from cardiovascular cause and from undetermined causes adjudicated by that committee.
aaTwo-sided p-value for the test of no difference. The significance level was 0.03224.1
bb2.2% ARR reported at 3 years in a prespecified supplementary analysis.3
ARR=absolute risk reduction; CI=confidence interval; CKD=chronic kidney disease; CV=cardiovascular; EAC=external adjudication committee; eGFR=estimated glomerular filtration rate; GLP-1 RA=glucagon-like peptide-1 receptor agonist; MACE=major adverse cardiovascular event; MI=myocardial infarction; RRR=relative risk reduction; SOC=standard of care; SLT-2i=sodium glucose co-transporter inhibitor; T2D=type 2 diabetes.
Significant reduction in all-cause mortality with Ozempic®1
For adults with T2D and CKD for a confirmatory secondary endpoint: time to first occurrence of all-cause mortality vs placebo when both were added to standard of care1,2

All cause death1,2,3
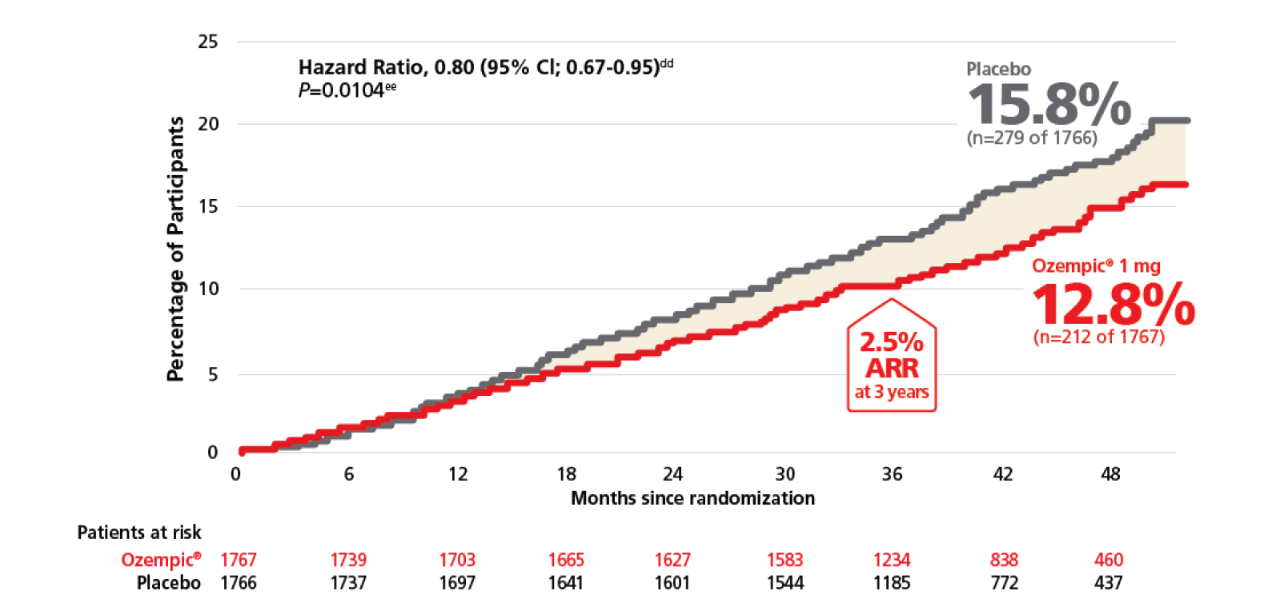
cc2.5%ARR reported at 3 years in pre-specified supplementary analysis.3
ddCox proportional hazards model with treatment as factor and stratified by baseline use of SGLT2-inhibitor at baseline (yes or no).1
eeTwo-sided p value for the test of no difference. The significance level was 0.03444.1
Ozempic® reduced the annual rate of change in eGFR1
For adults with T2D and CKD for a confirmatory secondary endpoint: observed mean change in eGFR over time (the annual rate of change in eGFR) vs placebo when both were added to standard of care1,2
Observed mean plot: eGFR(mL/min/1.73m2) by week in FLOW trial1,2,ff
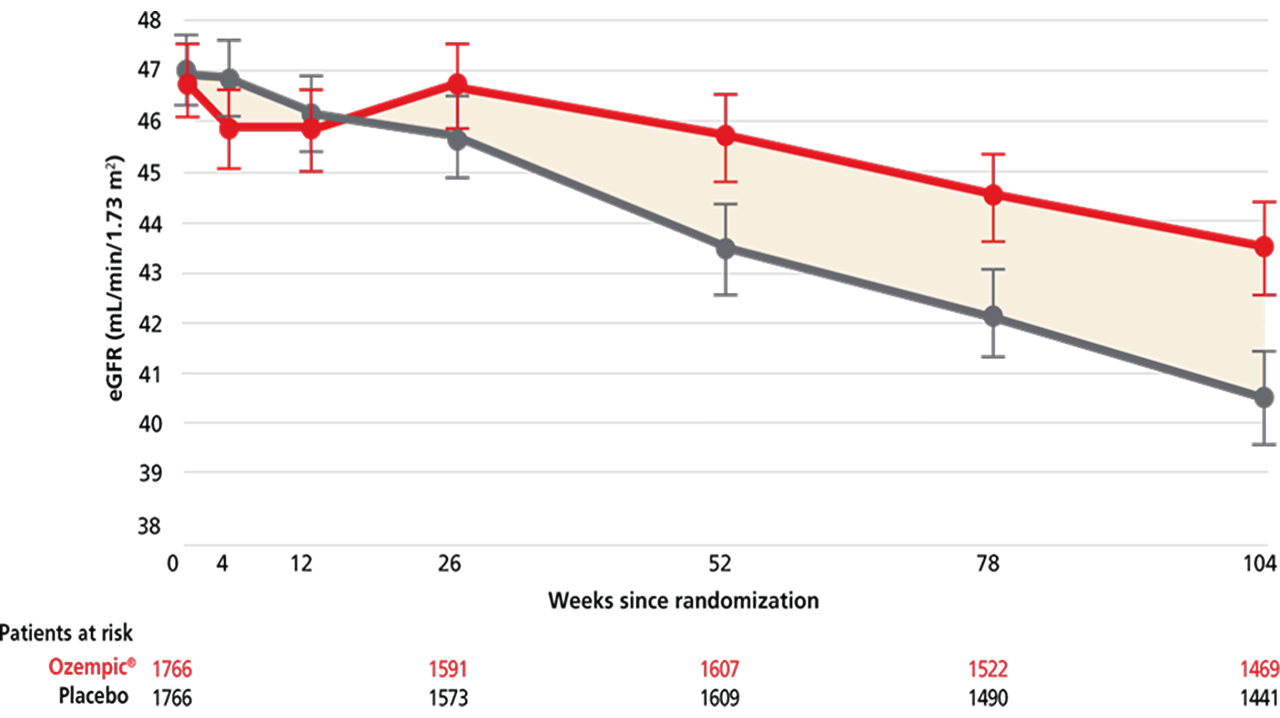
ffObserved data from the in-trial period until week 104. Error bars are +/- 1.96 *standard error of the mean eGFR, which was calculated using the CKD-EPI 2009 formula.1
For CKD benefit
Recommended Dosage for Patients with Type 2 Diabetes and Chronic Kidney Disease:
Initiate Ozempic® with a dosage of 0.25 mg once weekly for 4 weeks. After 4 weeks on the 0.25 mg, increase the dosage to 0.5 mg once weekly. Increase to the maintenance dosage, 1 mg once weekly, after at least 4 weeks on the 0.5 mg dosage.
More ways to help your patients with T2D
Important Safety Information for Ozempic®
WARNING: RISK OF THYROID C-CELL TUMORS
- In rodents, semaglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether Ozempic® causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined
- Ozempic® is contraindicated in patients with a personal or family history of MTC and in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of Ozempic® and inform them of symptoms of thyroid tumors (eg, a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with Ozempic®
Contraindications
- Ozempic® is contraindicated in patients with a personal or family history of MTC or in patients with MEN 2, and in patients with a hypersensitivity reaction to semaglutide or to any of the excipients in Ozempic®. Serious hypersensitivity reactions including anaphylaxis and angioedema have been reported with Ozempic®
Warnings and Precautions
- Risk of Thyroid C-Cell Tumors: Patients should be further evaluated if serum calcitonin is measured and found to be elevated or thyroid nodules are noted on physical examination or neck imaging
- Acute Pancreatitis: Acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with GLP-1 receptor agonists, including semaglutide. Observe patients carefully for signs and symptoms of pancreatitis (persistent severe abdominal pain, sometimes radiating to the back with or without vomiting). If pancreatitis is suspected, discontinue Ozempic® and initiate appropriate management
- Diabetic Retinopathy Complications: In a 2-year trial involving patients with type 2 diabetes and high cardiovascular risk, more events of diabetic retinopathy complications occurred in patients treated with Ozempic® (3.0%) compared with placebo (1.8%). The absolute risk increase for diabetic retinopathy complications was larger among patients with a history of diabetic retinopathy at baseline than among patients without a known history of diabetic retinopathy.
Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy. The effect of long-term glycemic control with semaglutide on diabetic retinopathy complications has not been studied. Patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy - Never Share an Ozempic® Pen Between Patients: Ozempic® pens must never be shared between patients, even if the needle is changed. Pen-sharing poses a risk for transmission of blood-borne pathogens
- Hypoglycemia: Patients receiving Ozempic® in combination with an insulin secretagogue (e.g., sulfonylurea) or insulin may have an increased risk of hypoglycemia, including severe hypoglycemia. Inform patients using these concomitant medications of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia
- Acute Kidney Injury Due to Volume Depletion: There have been postmarketing reports of acute kidney injury, in some cases requiring hemodialysis, in patients treated with semaglutide. The majority of reported events occurred in patients who experienced gastrointestinal reactions leading to dehydration such as nausea, vomiting, or diarrhea. Monitor renal function in patients reporting adverse reactions to Ozempic® that could lead to volume depletion, especially during dosage initiation and escalation
- Severe Gastrointestinal Adverse Reactions: Use of Ozempic® has been associated with gastrointestinal adverse reactions, sometimes severe. In Ozempic® clinical trials, severe gastrointestinal adverse reactions were reported more frequently among patients receiving Ozempic® (0.5 mg 0.4%, 1 mg 0.8%) than placebo (0%). Ozempic® is not recommended in patients with severe gastroparesis
- Hypersensitivity: Serious hypersensitivity reactions (e.g., anaphylaxis, angioedema) have been reported in patients treated with Ozempic®. If hypersensitivity reactions occur, discontinue use of Ozempic®; treat promptly per standard of care, and monitor until signs and symptoms resolve. Use caution in a patient with a history of angioedema or anaphylaxis with another GLP-1 receptor agonist
- Acute Gallbladder Disease: Acute events of gallbladder disease such as cholelithiasis or cholecystitis have been reported in GLP-1 receptor agonist trials and postmarketing. In placebo-controlled trials, cholelithiasis was reported in 1.5% and 0.4% of patients treated with Ozempic® 0.5 mg and 1 mg, respectively, and not reported in placebo-treated patients. If cholelithiasis is suspected, gallbladder studies and appropriate clinical follow-up are indicated
- Pulmonary Aspiration During General Anesthesia or Deep Sedation: Ozempic® delays gastric emptying. There have been rare postmarketing reports of pulmonary aspiration in patients receiving GLP-1 receptor agonists undergoing elective surgeries or procedures requiring general anesthesia or deep sedation who had residual gastric contents despite reported adherence to preoperative fasting recommendations. Instruct patients to inform healthcare providers prior to any planned surgeries or procedures if they are taking Ozempic®
Adverse Reactions
- The most common adverse reactions, reported in ≥5% of patients treated with Ozempic® are nausea, vomiting, diarrhea, abdominal pain, and constipation
Drug Interactions
- When initiating Ozempic®, consider reducing the dose of concomitantly administered insulin secretagogue (such as sulfonylureas) or insulin to reduce the risk of hypoglycemia
- Ozempic® causes a delay of gastric emptying and has the potential to impact the absorption of concomitantly administered oral medications, so caution should be exercised
Use in Specific Populations
- There are limited data with semaglutide use in pregnant women to inform a drug-associated risk for adverse developmental outcomes. Discontinue Ozempic® in women at least 2 months before a planned pregnancy due to the long washout period for semaglutide
Please click here for Ozempic® Prescribing Information, including Boxed Warning.
Indications and Usage
Ozempic® (semaglutide) injection 0.5 mg, 1 mg, or 2 mg is indicated:
- as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes
- to reduce the risk of major adverse cardiovascular (CV) events (CV death, nonfatal myocardial infarction, or nonfatal stroke) in adults with type 2 diabetes and established CV disease
- to reduce the risk of sustained eGFR decline, end-stage kidney disease, and cardiovascular death in adults with type 2 diabetes and chronic kidney disease
Important Safety Information for Ozempic®
WARNING: RISK OF THYROID C-CELL TUMORS
- In rodents, semaglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether Ozempic® causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined
- Ozempic® is contraindicated in patients with a personal or family history of MTC and in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of Ozempic® and inform them of symptoms of thyroid tumors (eg, a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with Ozempic®
Important Safety Information for Ozempic®
WARNING: RISK OF THYROID C-CELL TUMORS
- In rodents, semaglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether Ozempic® causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined
- Ozempic® is contraindicated in patients with a personal or family history of MTC and in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of Ozempic® and inform them of symptoms of thyroid tumors (eg, a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with Ozempic®
Contraindications
- Ozempic® is contraindicated in patients with a personal or family history of MTC or in patients with MEN 2, and in patients with a hypersensitivity reaction to semaglutide or to any of the excipients in Ozempic®. Serious hypersensitivity reactions including anaphylaxis and angioedema have been reported with Ozempic®
Warnings and Precautions
- Risk of Thyroid C-Cell Tumors: Patients should be further evaluated if serum calcitonin is measured and found to be elevated or thyroid nodules are noted on physical examination or neck imaging
- Acute Pancreatitis: Acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with GLP-1 receptor agonists, including semaglutide. Observe patients carefully for signs and symptoms of pancreatitis (persistent severe abdominal pain, sometimes radiating to the back with or without vomiting). If pancreatitis is suspected, discontinue Ozempic® and initiate appropriate management
- Diabetic Retinopathy Complications: In a 2-year trial involving patients with type 2 diabetes and high cardiovascular risk, more events of diabetic retinopathy complications occurred in patients treated with Ozempic® (3.0%) compared with placebo (1.8%). The absolute risk increase for diabetic retinopathy complications was larger among patients with a history of diabetic retinopathy at baseline than among patients without a known history of diabetic retinopathy.
Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy. The effect of long-term glycemic control with semaglutide on diabetic retinopathy complications has not been studied. Patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy - Never Share an Ozempic® Pen Between Patients: Ozempic® pens must never be shared between patients, even if the needle is changed. Pen-sharing poses a risk for transmission of blood-borne pathogens
- Hypoglycemia: Patients receiving Ozempic® in combination with an insulin secretagogue (e.g., sulfonylurea) or insulin may have an increased risk of hypoglycemia, including severe hypoglycemia. Inform patients using these concomitant medications of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia
- Acute Kidney Injury Due to Volume Depletion: There have been postmarketing reports of acute kidney injury, in some cases requiring hemodialysis, in patients treated with semaglutide. The majority of reported events occurred in patients who experienced gastrointestinal reactions leading to dehydration such as nausea, vomiting, or diarrhea. Monitor renal function in patients reporting adverse reactions to Ozempic® that could lead to volume depletion, especially during dosage initiation and escalation
- Severe Gastrointestinal Adverse Reactions: Use of Ozempic® has been associated with gastrointestinal adverse reactions, sometimes severe. In Ozempic® clinical trials, severe gastrointestinal adverse reactions were reported more frequently among patients receiving Ozempic® (0.5 mg 0.4%, 1 mg 0.8%) than placebo (0%). Ozempic® is not recommended in patients with severe gastroparesis
- Hypersensitivity: Serious hypersensitivity reactions (e.g., anaphylaxis, angioedema) have been reported in patients treated with Ozempic®. If hypersensitivity reactions occur, discontinue use of Ozempic®; treat promptly per standard of care, and monitor until signs and symptoms resolve. Use caution in a patient with a history of angioedema or anaphylaxis with another GLP-1 receptor agonist
- Acute Gallbladder Disease: Acute events of gallbladder disease such as cholelithiasis or cholecystitis have been reported in GLP-1 receptor agonist trials and postmarketing. In placebo-controlled trials, cholelithiasis was reported in 1.5% and 0.4% of patients treated with Ozempic® 0.5 mg and 1 mg, respectively, and not reported in placebo-treated patients. If cholelithiasis is suspected, gallbladder studies and appropriate clinical follow-up are indicated
- Pulmonary Aspiration During General Anesthesia or Deep Sedation: Ozempic® delays gastric emptying. There have been rare postmarketing reports of pulmonary aspiration in patients receiving GLP-1 receptor agonists undergoing elective surgeries or procedures requiring general anesthesia or deep sedation who had residual gastric contents despite reported adherence to preoperative fasting recommendations. Instruct patients to inform healthcare providers prior to any planned surgeries or procedures if they are taking Ozempic®
Adverse Reactions
- The most common adverse reactions, reported in ≥5% of patients treated with Ozempic® are nausea, vomiting, diarrhea, abdominal pain, and constipation
Drug Interactions
- When initiating Ozempic®, consider reducing the dose of concomitantly administered insulin secretagogue (such as sulfonylureas) or insulin to reduce the risk of hypoglycemia
- Ozempic® causes a delay of gastric emptying and has the potential to impact the absorption of concomitantly administered oral medications, so caution should be exercised
Use in Specific Populations
- There are limited data with semaglutide use in pregnant women to inform a drug-associated risk for adverse developmental outcomes. Discontinue Ozempic® in women at least 2 months before a planned pregnancy due to the long washout period for semaglutide
Please click here for Ozempic® Prescribing Information, including Boxed Warning.
Indications and Usage
Ozempic® (semaglutide) injection 0.5 mg, 1 mg, or 2 mg is indicated:
- as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes
- to reduce the risk of major adverse cardiovascular (CV) events (CV death, nonfatal myocardial infarction, or nonfatal stroke) in adults with type 2 diabetes and established CV disease
- to reduce the risk of sustained eGFR decline, end-stage kidney disease, and cardiovascular death in adults with type 2 diabetes and chronic kidney disease
References:
- Ozempic® [package insert]. Plainsboro, NJ: Novo Nordisk Inc.
- Perkovic V, Tuttle KR, Rossing P, et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N Engl J Med. 2024;391:109-121.
- Data on file. Novo Nordisk Inc; Plainsboro, NJ.
- Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601-609.
- Perkovic V, Tuttle KR, Rossing P, et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. Supplementary appendix. N Engl J Med. 2024;391:109-121.
- Rossing P, Baeres FMM, Bakris G, et al. The rationale, design and baseline data of FLOW, a kidney outcomes trial with once-weekly semaglutide in people with type 2 diabetes and chronic kidney disease. Nephrol Dial Transplant. 2023;38(9):2041-2051.

