Start early for glycemic control
Help your patients with T2D achieve their A1C goals with Ozempic® (semaglutide)1
Ozempic® vs Trulicity® and Ozempic® 1 mg vs 2 mg1
SUSTAIN 7:
vs Trulicity®2,3
In patients with type 2 diabetes on metformin
Superior results vs Trulicity®
Mean baseline A1C:

SUSTAIN FORTE:
Ozempic® 1 mg vs 2 mg1,4
In patients with type 2 diabetes on metformin ± sulfonylurea
Superior results with Ozempic®
2 mg dose
Mean baseline A1C:


Results for SUSTAIN 7 and SUSTAIN FORTE are based on sensitivity analyses of retrieved dropout population.
SUSTAIN 7: A 40-week, randomized, open-label, active-controlled trial in 1201 adult patients with type 2 diabetes on metformin, comparing Ozempic® 0.5 mg with Trulicity® 0.75 mg and Ozempic® 1 mg with Trulicity® 1.5 mg.2
SUSTAIN FORTE: A 40-week, randomized, active-controlled trial in 961 adult patients with type 2 diabetes on metformin with or without a sulfonylurea, comparing Ozempic® 1 mg with Ozempic® 2 mg.4
SUSTAIN 7:
vs Trulicity®2
In patients with type 2 diabetes on metformin
Superior results vs Trulicity®
Mean baseline A1C:

SUSTAIN FORTE:
Ozempic® 1 mg vs 2 mg1,4
In patients with type 2 diabetes on metformin ± sulfonylurea
Superior results with Ozempic®
2 mg dose
Mean baseline A1C:


SUSTAIN 7: Predefined secondary endpoint analyzed using post-hoc analysis of retrieved dropout population. Not controlled for multiplicity. Results are from a 40-week, randomized, open-label, active-controlled trial in 1201 adult patients with type 2 diabetes on metformin, comparing Ozempic® 0.5 mg with Trulicity® 0.75 mg and Ozempic® 1 mg with Trulicity® 1.5 mg.2,3
SUSTAIN FORTE: Predefined secondary endpoint based on analysis of retrieved dropout population. Values are dichotomized and denominator is the number of all randomized subjects. Due to statistical hierarchy, differences were not formally tested. Results are from a 40-week, randomized, active-controlled trial in 961 adult patients with type 2 diabetes on metformin with or without a sulfonylurea, comparing Ozempic® 1 mg with Ozempic® 2 mg.1,4
aAccording to the ADA, a reasonable A1C goal for many nonpregnant adults is less than 7%. However, a doctor may recommend a different goal based on age, other health conditions, and other factors.5
ADA=American Diabetes Association.

Adverse events ≥5% in SUSTAIN 72
SUSTAIN 7 was not designed to evaluate relative safety between Ozempic® and Trulicity®

AE=adverse event; GI=gastrointestinal; T2D=type 2 diabetes.
- In placebo-controlled trials, the most common adverse reactions reported in ≥5% of patients treated with Ozempic® are nausea, vomiting, diarrhea, abdominal pain, and constipation.1
- Because clinical trials are conducted under widely varying conditions, adverse-reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.1
- Comparator AE rates are not an adequate basis for comparison of safety between products.1

- In placebo-controlled trials, the most common adverse reactions reported in ≥5% of patients treated with Ozempic® are nausea, vomiting, diarrhea, abdominal pain, and constipation.1
- No new safety signals were identified. Gastrointestinal adverse reactions occurred more frequently among patients receiving Ozempic® 2 mg (34.0%) vs Ozempic® 1 mg (30.8%).4
- Incidence of severe hypoglycemia (level 3) was <1% for Ozempic® 1 mg and Ozempic® 2 mg in the SUSTAIN FORTE trial.4
Body weight change was studied as a secondary endpoint
Ozempic® is not indicated for weight loss.
SUSTAIN 4:
vs study-titrated Lantus®
In insulin-naïve adult patients with type 2
diabetes on metformin ± sulfonylurea
Mean change in A1C from baseline at Week 301,5
Mean baseline A1C:

26% of insulin patients titrated to goal by week 30. Mean daily insulin dose: 29 U/day
SUSTAIN FORTE:
Ozempic® 1 mg vs 2 mg
In patients with type 2 diabetes on
metformin ± sulfonylurea
Mean change in A1C from baseline at Week 401,4
Mean baseline A1C:


Results for SUSTAIN 4 and SUSTAIN FORTE are based on sensitivity analyses of retrieved dropout population.
SUSTAIN 4: Results are from a 30-week, randomized, open-label, active-controlled trial in 1089 insulin-naïve adult patients on metformin ± sulfonylurea with type 2 diabetes comparing Ozempic® 0.5 mg and Ozempic® 1 mg with Lantus®.6
SUSTAIN FORTE: Results are from a 40-week, randomized, active-controlled trial in 961 adult patients with type 2 diabetes on metformin with or without a sulfonylurea, comparing Ozempic® 1 mg with Ozempic® 2 mg.4
SUSTAIN 4:
vs study-titrated Lantus®
In insulin-naïve adult patients with type 2 diabetes on metformin ± sulfonylurea
Percent of patients who achieved A1C <7% at Week 301,6
Mean baseline A1C:

26% of insulin patients titrated to goal by week 30. Mean daily insulin dose: 29 U/day
SUSTAIN FORTE:
Ozempic® 1 mg vs 2 mg
In patients with type 2 diabetes on
metformin ± sulfonylurea
Percent of patients who achieved A1C <7% at Week 401,4
Mean baseline A1C:


SUSTAIN 4: Predefined secondary endpoint based on a post-hoc analysis of retrieved dropout population. Results are from a 30-week, randomized, open-label, active-controlled trial in 1089 insulin-naïve adult patients on metformin ± sulfonylurea with type 2 diabetes comparing Ozempic® 0.5 mg and Ozempic® 1 mg with Lantus®.1,6
SUSTAIN FORTE: Predefined secondary endpoint based on analysis of retrieved dropout population. Values are dichotomized and denominator is the number of all randomized subjects. Due to statistical hierarchy, differences were not formally tested. Results are from a 40-week, randomized, active-controlled trial in 961 adult patients with type 2 diabetes on metformin with or without a sulfonylurea, comparing Ozempic® 1 mg with Ozempic® 2 mg.1,4
ADA=American Diabetes Association.
Adverse events ≥5% in SUSTAIN 4
AEs occurring in ≥5% of participants treated with Ozempic® in SUSTAIN 46
SUSTAIN 4 was not designed to evaluate relative safety between Ozempic® and Lantus®


AE=adverse event; GI=gastrointestinal.
- In placebo-controlled trials, the most common adverse reactions reported in ≥5% of patients treated with Ozempic® are nausea, vomiting, diarrhea, abdominal pain, and constipation.1
- Because clinical trials are conducted under widely varying conditions, adverse-reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- Comparator AE rates are not an adequate basis for comparison of safety between products.
- Incidence of severe hypoglycemia was ≤1.5% across all placebo-controlled trials.1
- Incidence of severe hypoglycemia or blood glucose-confirmed hypoglycemia (% of patients) was 4% with Ozempic® 0.5 mg, 6% with Ozempic® 1 mg, and 11% with Lantus®.5,b
bDefined as an event requiring assistance of another person to actively administer carbohydrates or glucagon, or take other corrective actions or blood glucose-confirmed symptomatic hypoglycemia (plasma glucose ≤3.1 mmol/L [56 mg/dL]).6
Could Ozempic® help your patients who may
be at risk for a CV event?
SUSTAIN 5:
Add-on to basal insulin
In adult patients with type 2 diabetes on basal insulin ± metformin
Mean change in A1C from baseline at Week 301,7,8
Mean baseline A1C:

SUSTAIN FORTE:
Ozempic® 1 mg vs 2 mg
In patients with type 2 diabetes on metformin ± sulfonylurea
Mean change in A1C from baseline at Week 401,4
Mean baseline A1C:


Results for SUSTAIN 5 and SUSTAIN FORTE based on sensitivity analyses of retrieved dropout population.
SUSTAIN 5: Results are from a 30-week, randomized, double-blind, placebo-controlled, parallel-group trial in 397 adult patients with type 2 diabetes evaluating the addition of Ozempic® 0.5 mg and Ozempic® 1 mg to basal insulin ± metformin. Patients with A1C ≤8% at screening had their background basal insulin dose reduced by 20% at the start of the trial to limit potential risk of hypoglycemia.1,7
SUSTAIN FORTE: Results are from a 40-week, randomized, active-controlled trial in 961 adult patients with type 2 diabetes on metformin with or without a sulfonylurea, comparing Ozempic® 1 mg with Ozempic® 2 mg.4
MET=metformin.
SUSTAIN 5:
Add-on to basal insulin
In adult patients with type 2 diabetes on basal insulin ± metformin
Percent of patients who achieved A1C <7% at Week 301,7
Mean baseline A1C:

SUSTAIN FORTE:
Ozempic® 1 mg vs 2 mg
In patients with type 2 diabetes on metformin ± sulfonylurea
Percent of patients who achieved A1C <7% at Week 401,4
Mean baseline A1C:


SUSTAIN 5: Predefined secondary endpoint analyzed using post-hoc analysis of retrieved dropout population. Results are from a 30-week, randomized, double-blind, placebo-controlled, parallel-group trial in 397 adult patients with type 2 diabetes evaluating the addition of Ozempic® 0.5 mg and Ozempic® 1 mg to basal insulin ± metformin.1,7
SUSTAIN FORTE: Predefined secondary endpoint based on analysis of retrieved dropout population. Values are dichotomized and denominator is the number of all randomized subjects. Due to statistical hierarchy, differences were not formally tested. Results are from a 40-week, randomized, active-controlled trial in 961 adult patients with type 2 diabetes on metformin with or without a sulfonylurea, comparing Ozempic® 1 mg with Ozempic® 2 mg.1,4
ADA=American Diabetes Association; MET=metformin.
SUSTAIN 5:
In adult patients with type 2 diabetes on basal insulin ± metformin
Secondary endpoint: mean change in FPG at Week 301,c
Baseline:

SUSTAIN 5:
In adult patients with type 2 diabetes on basal insulin ± metformin
Mean change in PPG at Week 309
Baseline:


Mean change in 90-minute PPG from baseline at Week 30 was not a predefined endpoint. Post-hoc analysis of absolute PPG means was calculated from the 7-point SMPG profile.
The clinical relevance of FPG and PPG is unknown.
Patients with A1C ≤8% at screening had their background basal insulin dose reduced by 20% at the start of the trial to limit potential risk of hypoglycemia.7

Results are from a 30-week, randomized, double-blind, placebo-controlled, parallel-group trial in 397 adult patients with type 2 diabetes evaluating the addition of Ozempic® 0.5 mg and Ozempic® 1 mg to basal insulin ± metformin.7
cPredefined secondary endpoint using post-hoc analysis of retrieved dropout population; not adjusted for multiplicity.7
FPG=fasting plasma glucose; MET=metformin; PPG=postprandial glucose; SMPG=self-monitoring of plasma glucose.
Adverse events ≥5% in SUSTAIN 5
AEs occurring in ≥5% of participants treated with Ozempic® in SUSTAIN 57,9


AE=adverse event; GI=gastrointestinal.
- Incidence of severe hypoglycemia was ≤1.5% across all placebo-controlled trials.1
- Incidence of severe hypoglycemia or blood glucose-confirmed hypoglycemia (% of patients) was7,9:
- 8.3% with Ozempic® 0.5 mg + basal insulin ± MET
- 10.7% with Ozempic® 1 mg + basal insulin ± MET
- 5.3% with placebo + basal insulin ± MET
- Patients receiving Ozempic® in combination with an insulin secretagogue (eg, sulfonylurea) or insulin may have an increased risk of hypoglycemia, including severe hypoglycemia.1

SUSTAIN 9: add-on to SGLT-2i in adult patients with type 2 diabetes on SGLT-2i with or
without MET/SU needing additional glycemic control10
Primary endpoint: mean change in
A1C from baseline at Week 3010,11,d
Mean baseline A1C:



Consider Ozempic® for patients on SGLT-2i who may need additional
glycemic control and who may be concerned about weight10

Results based on sensitivity analyses of retrieved dropout population.
SUSTAIN 9: Results are from a 30-week, multinational, randomized, double-blind, parallel-group, placebo-controlled trial to evaluate the efficacy and safety of adding Ozempic® 1 mg to SGLT-2i ± metformin/sulfonylurea vs SGLT-2i + placebo ± metformin/sulfonylurea. Patients were on SGLT-2i for an average of approximately 46 weeks at randomization.10
dEstimated mean.
SUSTAIN 9: add-on to SGLT-2i in adult patients with type 2 diabetes on SGLT-2i with or
without MET/SU needing additional glycemic control10
Secondary endpoint: mean change
in body weight from baseline at
Week 3010,11,d


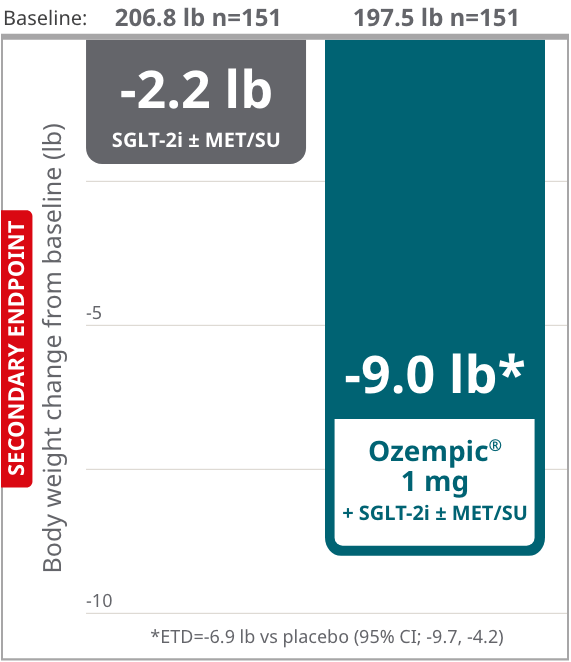
Consider Ozempic® for patients on SGLT-2i who may need additional glycemic control and who may be concerned about weight10


Results based on sensitivity analyses of retrieved dropout population.
SUSTAIN 9: Results are from a 30-week, multinational, randomized, double-blind, parallel-group, placebo-controlled trial to evaluate the efficacy and safety of adding Ozempic® 1 mg to SGLT-2i ± metformin/sulfonylurea vs SGLT-2i + placebo ± metformin/sulfonylurea. Patients were on SGLT-2i for an average of approximately 46 weeks at randomization.10
dEstimated mean.
SUSTAIN 9: add-on to SGLT-2i in adult patients with type 2 diabetes on SGLT-2i with or
without MET/SU needing additional glycemic control10
Secondary endpoint: percent of
patients who achieved A1C <7% at
Week 3010,11



Consider Ozempic® for patients on SGLT-2i who may need additional glycemic
control and who may be concerned about weight10

Results based on sensitivity analyses of retrieved dropout population.
SUSTAIN 9: Results are from a 30-week, multinational, randomized, double-blind, parallel-group, placebo-controlled trial to evaluate the efficacy and safety of adding Ozempic® 1 mg to SGLT-2i ± metformin/sulfonylurea vs SGLT-2i + placebo ± metformin/sulfonylurea. Patients were on SGLT-2i for an average of approximately 46 weeks at randomization.10
dEstimated mean.
Adverse events ≥5% in SUSTAIN 9
AEs occurring in ≥5% of participants treated with Ozempic® in SUSTAIN 99,10

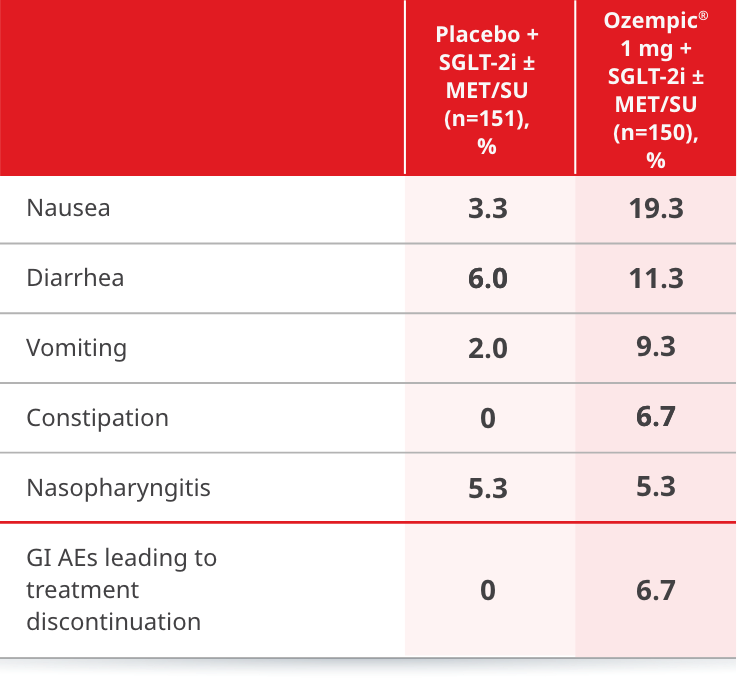

eAmerican Diabetes Association classified, including hypoglycemia episodes classified as severe, documented symptomatic, asymptomatic, probably symptomatic, and pseudo-hypoglycemia.
ETD=estimated treatment difference; MET=metformin; SGLT-2i=sodium-glucose cotransporter-2 inhibitor; SU=sulfonylurea.
- In placebo-controlled trials, the most common adverse reactions reported in ≥5% of patients treated with Ozempic® are nausea, vomiting, diarrhea, abdominal pain, and constipation.1
- Incidence of severe hypoglycemia was ≤1.5% across all placebo-controlled trials.1
- Incidence of hypoglycemia (% of patients) was 11.3% with Ozempic® 1 mg + SGLT-2i ± MET/SU and 2.0% with placebo + SGLT-2i ± MET/SU.10,e
More ways to help your patients with T2D
CV=cardiovascular; T2D=type 2 diabetes.
STUDY DESIGNS
SUSTAIN 7: head-to-head vs Trulicity® (dulaglutide)2
Study design: 40-week, multinational, multicenter, randomized, open-label, 4-armed, pairwise, active-controlled, parallel-group trial to evaluate the efficacy and safety of Ozempic® vs dulaglutide.
Patients: A total of 1201 adult patients with type 2 diabetes inadequately controlled on metformin were randomized to receive Ozempic® 0.5 mg (n=301), Ozempic® 1 mg (n=300), dulaglutide 0.75 mg (n=299), or dulaglutide 1.5 mg (n=299) once weekly.
Primary endpoint: mean change in A1C from baseline at Week 40.
Secondary endpoints: mean change in body weight from baseline at Week 40; proportion of patients achieving A1C <7% at Week 40.
SUSTAIN 4: head-to-head vs Lantus® (insulin glargine U-100)6
Study design: 30-week, randomized, open-label, active-controlled, parallel-group, multinational, multicenter trial to evaluate the efficacy and safety of Ozempic® vs insulin glargine U‑100.
Patients: A total of 1089 insulin-naïve adult patients with type 2 diabetes inadequately controlled on metformin alone (48%) or in combination with a sulfonylurea (51%) were randomized to receive once-weekly Ozempic® 0.5 mg (n=362), once-weekly Ozempic® 1 mg (n=360), or once-daily insulin glargine U-100 (n=360). Patients assigned to insulin glargine had a baseline mean A1C of 8.1% and were started on a dose of 10 units once daily. Insulin glargine dose adjustments occurred throughout the trial period based on self-measured fasting plasma glucose before breakfast, targeting 71 to <100 mg/dL. In addition, investigators could titrate insulin glargine based on their discretion between study visits. Twenty-six percent of patients had been titrated to goal by the primary endpoint at Week 30, at which time the mean daily insulin dose was 29 units per day.
Primary endpoint: mean change in A1C from baseline at Week 30.
Secondary endpoints: mean change in body weight from baseline at Week 30; proportion of patients achieving A1C <7% at Week 30.
SUSTAIN 5: as an add-on to basal insulin vs placebo1,7
Study design: 30-week, randomized, double-blind, placebo-controlled, parallel-group, multinational, multicenter trial to compare the efficacy and safety of Ozempic® in combination with basal insulin vs volume-matched placebo in combination with basal insulin.
Patients: A total of 397 adult patients inadequately controlled on basal insulin with or without metformin were randomized to once-weekly Ozempic® 0.5 mg (n=132), Ozempic® 1 mg (n=131), or placebo (n=133). Randomization was stratified according to A1C at screening. Patients with A1C ≤8% at screening reduced the insulin dose by 20% at the start of the trial to reduce the risk of hypoglycemia.
Primary endpoint: mean change in A1C from baseline at Week 30.
Secondary endpoints: mean change in body weight from baseline at Week 30; proportion of patients achieving A1C <7% at Week 30; change in mean fasting plasma glucose (FPG) at Week 30.
SUSTAIN FORTE: Ozempic® 1 mg vs 2 mg4
Study design: 40-week, randomized, active-controlled, parallel-group, double-blind, phase 3B efficacy and safety trial of Ozempic® 2 mg vs Ozempic® 1 mg in patients with type 2 diabetes in need of treatment intensification.
Patients: A total of 961 adult patients with inadequately controlled type 2 diabetes (A1C 8.0%-10.0%) on metformin with or without a sulfonylurea were randomized 1:1 to 2 mg (n=480) or 1 mg (n=481) of once-weekly Ozempic®.
Primary endpoint: mean change in A1C from baseline at Week 40.
Secondary endpoints: mean change in body weight from baseline at Week 40; proportion of patients achieving A1C <7.0% at Week 40.
SUSTAIN 9: Ozempic® as an add-on to SGLT-2i ± MET/SU10
Study design: 30-week, multinational, randomized, double-blind, parallel-group, placebo-controlled trial evaluating the efficacy and safety of adding Ozempic® 1 mg to SGLT-2i ± metformin/sulfonylurea vs SGLT-2i + placebo ± metformin/sulfonylurea. Patients were on SGLT-2i for an average of approximately 46 weeks at randomization.
Patients: A total of 302 adult patients with type 2 diabetes inadequately controlled on SGLT-2i ± metformin/sulfonylurea were randomized to receive Ozempic® 1 mg (n=151) once weekly or volume-matched placebo (n=151) as an add-on to SGLT-2i ± metformin/sulfonylurea.
Primary endpoint: mean change in A1C from baseline at Week 30.
Secondary endpoints: mean change in body weight from baseline at Week 30; proportion of patients achieving A1C <7% at Week 30.
Important Safety Information for Ozempic® (semaglutide) injection
WARNING: RISK OF THYROID C-CELL TUMORS
- In rodents, semaglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether Ozempic® causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined
- Ozempic® is contraindicated in patients with a personal or family history of MTC and in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of Ozempic® and inform them of symptoms of thyroid tumors (eg, a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with Ozempic®
Indications and Usage
Ozempic® (semaglutide) injection 0.5 mg, 1 mg, or 2 mg is indicated:
- as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes
- to reduce the risk of major adverse cardiovascular (CV) events (CV death, nonfatal myocardial infarction, or nonfatal stroke) in adults with type 2 diabetes and established CV disease
- to reduce the risk of sustained eGFR decline, end-stage kidney disease, and cardiovascular death in adults with type 2 diabetes and chronic kidney disease
Important Safety Information cont.
Contraindications
- Ozempic® is contraindicated in patients with a personal or family history of MTC or in patients with MEN 2, and in patients with a hypersensitivity reaction to semaglutide or to any of the excipients in Ozempic®. Serious hypersensitivity reactions including anaphylaxis and angioedema have been reported with Ozempic®
Warnings and Precautions
- Risk of Thyroid C-Cell Tumors: Patients should be further evaluated if serum calcitonin is measured and found to be elevated or thyroid nodules are noted on physical examination or neck imaging
- Acute Pancreatitis: Acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with GLP-1 receptor agonists, including semaglutide. Observe patients carefully for signs and symptoms of pancreatitis (persistent severe abdominal pain, sometimes radiating to the back with or without vomiting). If pancreatitis is suspected, discontinue Ozempic® and initiate appropriate management
- Diabetic Retinopathy Complications: In a 2-year trial involving patients with type 2 diabetes and high cardiovascular risk, more events of diabetic retinopathy complications occurred in patients treated with Ozempic® (3.0%) compared with placebo (1.8%). The absolute risk increase for diabetic retinopathy complications was larger among patients with a history of diabetic retinopathy at baseline than among patients without a known history of diabetic retinopathy.
Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy. The effect of long-term glycemic control with semaglutide on diabetic retinopathy complications has not been studied. Patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy - Never Share an Ozempic® Pen Between Patients: Ozempic® pens must never be shared between patients, even if the needle is changed. Pen-sharing poses a risk for transmission of blood-borne pathogens
- Hypoglycemia: Patients receiving Ozempic® in combination with an insulin secretagogue (e.g., sulfonylurea) or insulin may have an increased risk of hypoglycemia, including severe hypoglycemia. Inform patients using these concomitant medications of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia
- Acute Kidney Injury Due to Volume Depletion: There have been postmarketing reports of acute kidney injury, in some cases requiring hemodialysis, in patients treated with semaglutide. The majority of reported events occurred in patients who experienced gastrointestinal reactions leading to dehydration such as nausea, vomiting, or diarrhea. Monitor renal function in patients reporting adverse reactions to Ozempic® that could lead to volume depletion, especially during dosage initiation and escalation
- Severe Gastrointestinal Adverse Reactions: Use of Ozempic® has been associated with gastrointestinal adverse reactions, sometimes severe. In Ozempic® clinical trials, severe gastrointestinal adverse reactions were reported more frequently among patients receiving Ozempic® (0.5 mg 0.4%, 1 mg 0.8%) than placebo (0%). Ozempic® is not recommended in patients with severe gastroparesis
- Hypersensitivity: Serious hypersensitivity reactions (e.g., anaphylaxis, angioedema) have been reported in patients treated with Ozempic®. If hypersensitivity reactions occur, discontinue use of Ozempic®; treat promptly per standard of care, and monitor until signs and symptoms resolve. Use caution in a patient with a history of angioedema or anaphylaxis with another GLP-1 receptor agonist
- Acute Gallbladder Disease: Acute events of gallbladder disease such as cholelithiasis or cholecystitis have been reported in GLP-1 receptor agonist trials and postmarketing. In placebo-controlled trials, cholelithiasis was reported in 1.5% and 0.4% of patients treated with Ozempic® 0.5 mg and 1 mg, respectively, and not reported in placebo-treated patients. If cholelithiasis is suspected, gallbladder studies and appropriate clinical follow-up are indicated
- Pulmonary Aspiration During General Anesthesia or Deep Sedation: Ozempic® delays gastric emptying. There have been rare postmarketing reports of pulmonary aspiration in patients receiving GLP-1 receptor agonists undergoing elective surgeries or procedures requiring general anesthesia or deep sedation who had residual gastric contents despite reported adherence to preoperative fasting recommendations. Instruct patients to inform healthcare providers prior to any planned surgeries or procedures if they are taking Ozempic®
Adverse Reactions
- The most common adverse reactions, reported in ≥5% of patients treated with Ozempic® are nausea, vomiting, diarrhea, abdominal pain, and constipation
Drug Interactions
- When initiating Ozempic®, consider reducing the dose of concomitantly administered insulin secretagogue (such as sulfonylureas) or insulin to reduce the risk of hypoglycemia
- Ozempic® causes a delay of gastric emptying and has the potential to impact the absorption of concomitantly administered oral medications, so caution should be exercised
Use in Specific Populations
- There are limited data with semaglutide use in pregnant women to inform a drug-associated risk for adverse developmental outcomes. Discontinue Ozempic® in women at least 2 months before a planned pregnancy due to the long washout period for semaglutide
Please click here for Ozempic® Prescribing Information, including Boxed Warning.
References:
- Ozempic® [package insert]. Plainsboro, NJ: Novo Nordisk Inc.
- Pratley RE, Aroda VR, Lingvay I, et al.; on behalf of the SUSTAIN 7 investigators. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6(4):275-286. doi:10.1016/S2213-8587(18)30024-X
- Pratley RE, Aroda VR, Lingvay I, et al.; on behalf of the SUSTAIN 7 investigators. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial (supplemental appendix). Lancet Diabetes Endocrinol. 2018;6(4):275-286. doi:10.1016/S2213-8587(18)30024-X
- Frías JP, Auerbach P, Bajaj HS, et al. Efficacy and safety of once-weekly semaglutide 2.0 mg versus 1.0 mg in patients with type 2 diabetes (SUSTAIN FORTE): a double-blind, randomised, phase 3B trial. Lancet Diabetes Endocrinol. 2021;9(9):563-574. doi: 10.1016/S2213-8587(21)00174-1
- American Diabetes Association Professional Practice Committee. Glycemic goals and hypoglycemia: standards of care in diabetes—2025. Diabetes Care. 2025;48(suppl 1):S128-S145.
- Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naïve patients with type 2 diabetes (SUSTAIN 4): a randomized, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017:5(5):355-366. doi:10.1016/S2213-8587(17)30085-2
- Rodbard HW, Lingvay I, Reed J, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab. 2018;103(6):2291-2301. doi:10.1210/jc.2018-00070
- Rodbard HW, Lingvay I, Reed J, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial (supplemental appendix). J Clin Endocrinol Metab. 2018;103(6):2291-2301. doi:10.1210/jc.2018-00070
- Data on file. Novo Nordisk Inc.; Plainsboro, NJ.
- Zinman B, Bhosekar V, Busch R, et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7(5):356-367. doi:10.1016/S2213-8587(19)30066-X
- Zinman B, Bhosekar V, Busch R, et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial (supplemental appendix). Lancet Diabetes Endocrinol. 2019;7(5):356-367. doi:10.1016/S2213-8587(19)30066-X