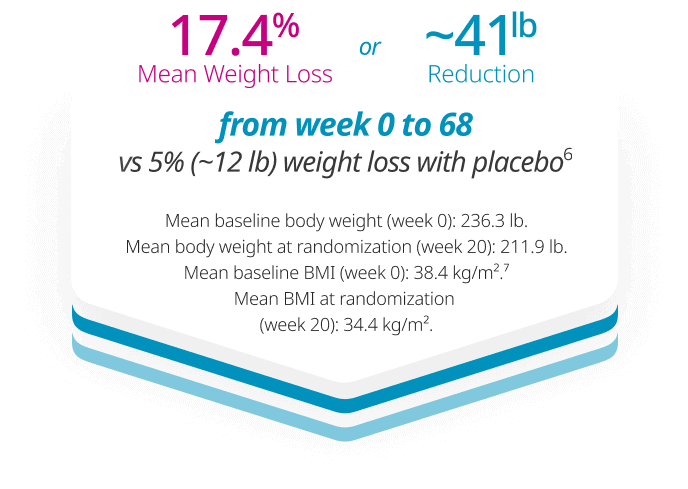In addition to diet and exercise, to reduce risk of MACE (cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke) in adults with established CVD and either overweight or obesity, and for chronic weight management in patients with obesity ≥12 years and adults with overweight with at least one weight-related comorbidity. Click for Limitations of Use.
Efficacy & Safety
Chronic Weight Management
In adults with obesity or overweight with at least one weight-related comorbidity, along with diet and exercise,
Significant weight loss that lasts1
Co-primary end point: Mean change in body weight (%) from week 0 to week 1041
During the trial, 13% of patients in the Wegovy® arm discontinued treatment compared with 27% in the placebo arm.
Missing data were imputed from retrieved subjects of the same randomized treatment arm (RD-MI).
*p<0.0001 (unadjusted 2-sided) for superiority.
†Difference from placebo was -12.6%.
Patients taking Wegovy® achieved1

BMI, body mass index.
STEP 5: A 104-week trial of 304 adults with obesity (BMI ≥30 kg/m2) or overweight (BMI 27 kg/m2-29.9 kg/m2) and ≥1 weight-related comorbidity, randomized 1:1 to Wegovy® or placebo, both with a reduced-calorie diet and increased physical activity. Discontinuation rate: Wegovy®=13%, placebo=27%.1 Click to see full study design below.
In adults with obesity or overweight with at least one weight-related comorbidity, along with diet and exercise,
With Wegovy®, the majority of patients maintained significant weight loss at 2 years1
≥20% weight loss maintained with Wegovy® by one-third of patients at 2 years1‡§
Observed weight loss at 104 weeks1

Supportive secondary end point
~1 out of 3 Wegovy® patients achieved1‡

‡Supportive secondary end points were not included in the statistical testing hierarchy and, as such, not controlled for multiplicity.
Weight loss in pounds (lb) calculated as 5%, 10%, 15%, and 20% of mean baseline body weight of Wegovy® and placebo patients.
§Observed data include only patients who had a body weight assessment at week 104 (144 of 152 for Wegovy® arm and 128 of 152 for placebo arm) and do not include all randomized patients.1
In adults with obesity or overweight with at least one weight-related comorbidity, along with diet and exercise,
Benefits beyond the scale® with Wegovy®
Patients taking Wegovy® 2.4 mg experienced improvements across most cardiometabolic risk factors1
Confirmatory secondary end points:
Change from baseline to week 104 in1:
Waist circumference:
5.7-inch reduction
2-inch reduction with placebo
Systolic blood pressure:
5.7 mm Hg reduction
1.6 mm Hg reduction with placebo
Supportive secondary end points: Change from baseline to week 104 in1:
Lipid profile:
Total cholesterol
3.3% reduction
1.4% increase with placebo
LDL
6.1% reduction
2.7% reduction with placebo
HDL
9.6% increase
8.1% increase with placebo
Triglycerides
19% reduction
3.7% increase with placebo
Diastolic blood pressure:
4.4 mm Hg reduction
0.8 mm Hg reduction with placebo
A1c:
0.4% reduction
0.1% reduction with placebo
Safety end point: Change in1:
Heart rate:
3.3 bpm increase
0.8 bpm reduction with placebo
Wegovy® is not indicated to treat hypertension, type 2 diabetes, or dyslipidemia.
The supportive secondary efficacy end points were not included in the statistical testing hierarchy and, as such, the analyses were not adjusted for multiplicity.
A1c, glycated hemoglobin; bpm, beats per minute; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Study design1
STEP 5: A 104-week trial of 304 adults with obesity (BMI ≥30 kg/m2) or with overweight (BMI 27 kg/m2-29.9 kg/m2) and at least one weight-related comorbid condition, such as dyslipidemia or hypertension, cardiovascular disease, or obstructive sleep apnea; patients with diabetes mellitus were excluded. Patients were randomized 1:1 to once-weekly Wegovy® 2.4 mg or placebo (with a 16-week dose escalation), both in conjunction with a reduced-calorie diet (~500 kcal/day deficit) and increased physical activity (recommended to a minimum of 150 min/week).
Co-primary end points1:
- Mean percentage change in body weight from baseline to week 104
- Percentage of patients achieving ≥5% weight loss from baseline to week 104
Relevant confirmatory secondary end points1:
- Percentage of patients achieving ≥10% weight loss from baseline to week 104
- Percentage of patients achieving ≥15% weight loss from baseline to week 104
- Change in waist circumference from baseline to week 104
- Change in systolic blood pressure from baseline to week 104
Relevant supportive secondary end points1:
- Percentage of patients achieving ≥20% weight loss from baseline to week 104
- Change in A1c from baseline to week 104
- Change in diastolic blood pressure from baseline to week 104
- Change in lipids‖ from baseline to week 104
‖LDL, HDL, triglycerides, and total cholesterol.
WeGoTogether® Real-World Study
Randomized clinical trials (RCTs)
- Evaluate the safety and efficacy of a treatment in specific populations under controlled conditions
- Prospective designs with prespecified, well-defined inclusion/exclusion criteria, outcomes, and end points
- Patients are randomly assigned to treatment or comparator
Limitations:
- Tightly controlled conditions and inclusion/exclusion criteria of RCTs may limit generalizability to real-world conditions or clinical populations
- Can be expensive and time-consuming
Randomized clinical trials are designed to show causality2,3
Real-world observational studies
- Use data from routine clinical practice
- Capture outcomes across broad patient populations
- Offer insight into how treatments perform outside of a controlled clinical trial setting
- Should be viewed as complementary to clinical trial data
Limitations:
- Susceptible to bias and confounding due to factors such as lack of randomization, missing or duplicative data, coding inaccuracies, and data variability reflecting routine clinical practice
- The presence of a pharmacy claim does not necessarily reflect actual drug utilization by the patient
- The approval of Wegovy® in 2021 resulted in a relatively short duration of follow-up, limiting the assessment of long-term outcomes, and the COVID-19 pandemic may have impacted data
Real-world studies evaluate associations and cannot determine causality3,4
In a retrospective, observational study of US adults with obesity,
Patients in a support program reported 21.2% weight loss with Wegovy®5


is a free behavior change program that is designed to help adults start and stay on Wegovy®. Patients get information on co-pay cards, 1:1 coaching, and tools to help them reframe behavior and adhere to their treatment regimen
Get patients started at Wegovy.com
Patients are advised to consult their HCP about treatment-related questions.

¶Note: 296/7,604 (4%) patients had a reported weight at 2 years.
Limitations: This study used descriptive methods, and no formal statistical comparisons were made. Data were self-reported including weight and height, and self-reported enrollment date (index) in the WeGoTogether® program may not reflect Wegovy® initiation. Baseline demographics were limited, and adherence data and clinical characteristics (such as comorbidities and concomitant medication use including obesity medications, GLP-1 RA, or GIP/GLP-1 RA) were unavailable. While self-reported dosage data were available, they could not be analyzed due to inconsistency and missing data.
Results should be interpreted in the context of limitations of this study.
Study design
WeGoTogether® Real-World Weight Outcomes: A retrospective observational study of US adults with obesity (BMI ≥30 kg/m2) treated with Wegovy® and enrolled in the WeGoTogether® patient support program evaluating patient-reported weight data. Among the 7,604 patients with obesity, 296 patients had reported weight values 24 months post index (±30 days).
Time periods and definitions: Study period: June 4, 2021 to April 10, 2025; index date: Reported date of enrollment (first injection start date) in the WeGoTogether® program.
End point:
- Mean percent weight change at 2 years
GIP, glucose-dependent insulinotropic polypeptide; GLP-1 RA, glucagon-like peptide-1 receptor agonist; HCP, health care professional.
In adults with obesity or overweight with at least one weight-related comorbidity, along with diet and exercise,
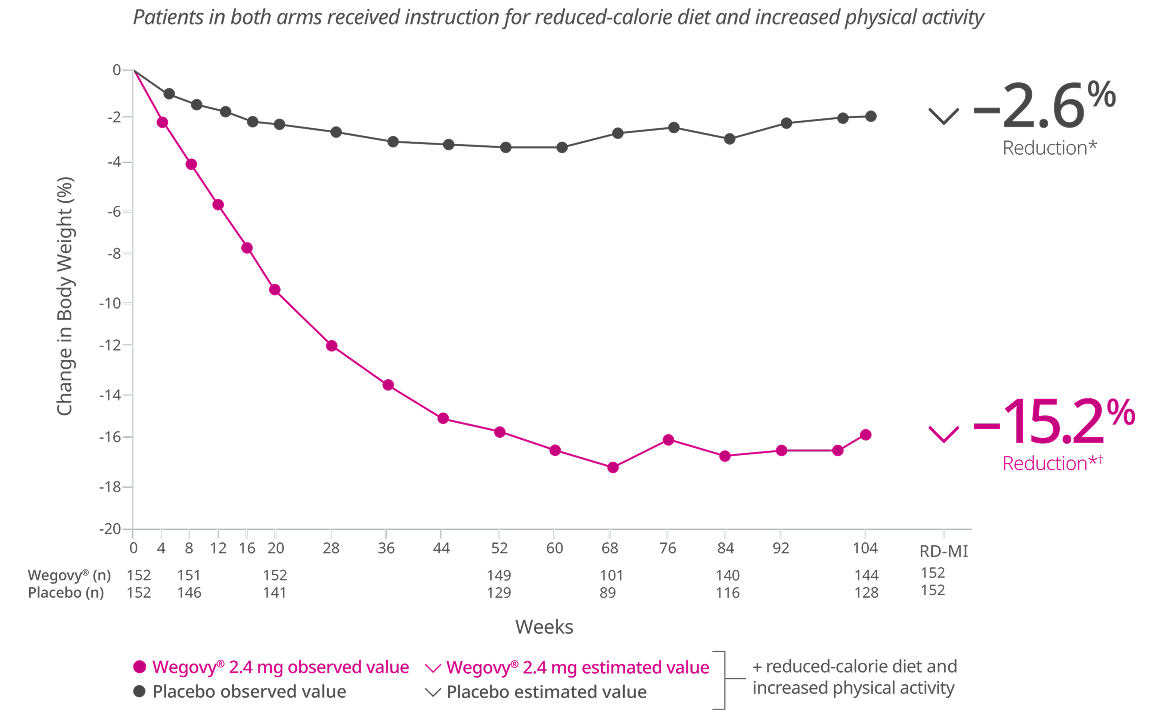
Patients who stayed on Wegovy® achieved sustained results against weight regain6
After a 20-week run-in period, patients who continued taking Wegovy® lost more weight and kept it off 6
Primary end point: Mean change in body weight (%) from randomization (week 20) to week 686
Randomized patients (shown) do not include 99 patients who discontinued during the 20-week run-in period.
Missing data were imputed from retrieved subjects of the same randomized treatment arm (RD-MI).
*p<0.001 (unadjusted 2-sided) for superiority, controlled for multiplicity.
†Difference from placebo was -14.8%.
Supportive secondary end point
Patients taking Wegovy® achieved6‡
‡Supportive secondary end points were not included in the statistical testing hierarchy and, as such, not controlled for multiplicity.
BMI, body mass index.
Study design6
STEP 4: A 68-week trial that enrolled 902 adults with obesity (BMI ≥30 kg/m2) or with overweight (BMI 27 kg/m2-29.9 kg/m2) and at least one weight-related comorbid condition, such as treated or untreated dyslipidemia or hypertension; patients with type 2 diabetes mellitus were excluded. All patients received once-weekly Wegovy® during the run-in period of 20 weeks, which included 16 weeks of dose escalation. 803 patients achieved Wegovy® 2.4 mg dose and were then randomized in a 2:1 ratio to either continue on Wegovy® or receive placebo. All patients received instruction for a reduced-calorie diet (~500 kcal/day deficit) and increased physical activity counseling (recommended to a minimum of 150 min/week) through the trial.
Primary end point6,7:
- Mean percentage change in body weight from randomization (week 20) to week 68
Relevant confirmatory secondary end points6,7:
- Change in waist circumference
- Change in systolic blood pressure
Relevant supportive secondary end points6,7:
- Change in body weight (%) from week 0 to week 68
In adults with obesity or overweight with at least one weight-related comorbidity, along with diet and exercise,
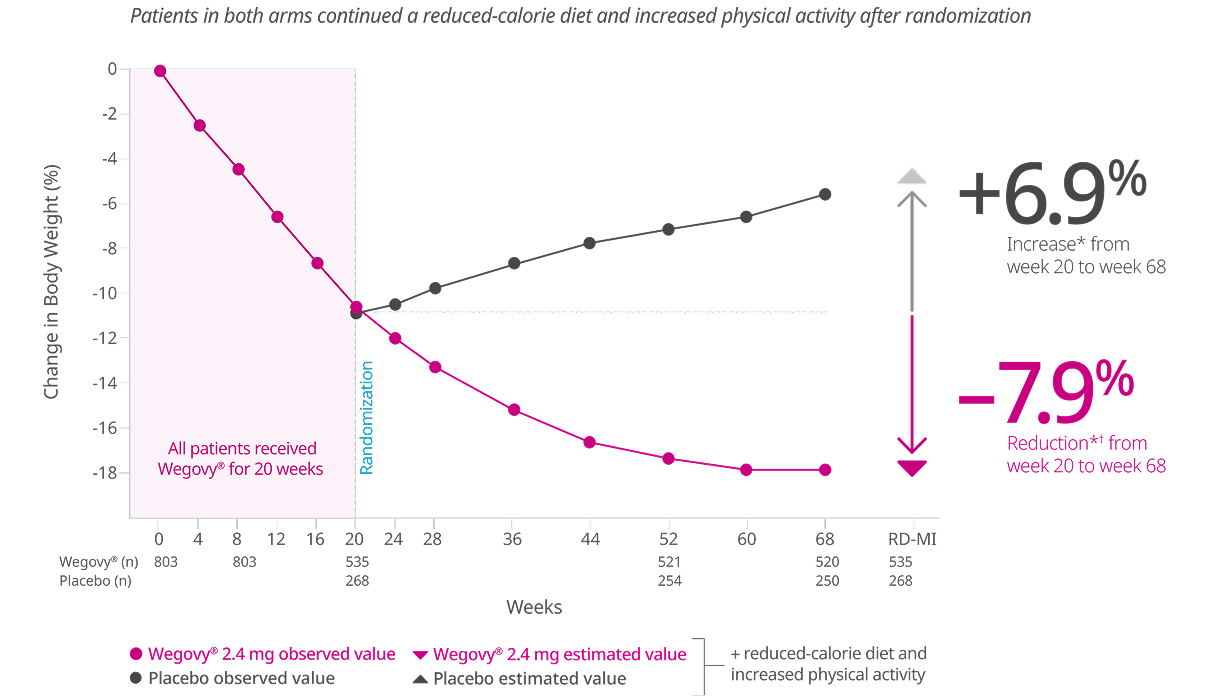
With the power of Wegovy®, patients achieved significant weight loss that started early6,8
Co-primary end point: Mean change in body weight (%) from week 0 to week 686,8
Missing data were imputed from retrieved subjects of the same randomized treatment arm (RD-MI).
During the trial, 17% of patients in the Wegovy® arm discontinued treatment compared with 22% in the placebo arm.
*p<0.0001 (unadjusted 2-sided) for superiority.
†Difference from placebo was -12.4%.
Patients taking Wegovy® achieved6,8

BMI, body mass index.
STEP 1: A 68-week trial of 1,961 adults with obesity or with overweight and at least one weight-related comorbidity randomized 2:1 to Wegovy® or placebo. Discontinuation rate: Wegovy®=17%, placebo=22%. Click to see full study design below.
In adults with obesity or overweight with at least one weight-related comorbidity, along with diet and exercise,
With Wegovy®, patients achieved 5%-20% or greater weight loss5,6,8
The majority of patients taking Wegovy® achieved clinically meaningful weight loss
Estimated weight loss at 68 weeks

Supportive secondary end point5,8
~1 out of 3 Wegovy® patients achieved‡

‡Supportive secondary end points were not included in the statistical testing hierarchy and, as such, not controlled for multiplicity. These data are from the cumulative frequency distribution for change in body weight.
Weight loss in pounds (lb) calculated as 5%, 10%, 15%, and 20% of mean baseline body weight of Wegovy® and placebo patients.
In adults with obesity or overweight with at least one weight-related comorbidity, along with diet and exercise,
Benefits beyond the scale® with Wegovy® from baseline to week 68
Wegovy® 2.4 mg demonstrated improvements in cardiometabolic risk factors at week 68
Confirmatory secondary end points:
Change in6,8§:
Waist circumference:
5.3-inch reduction
1.6-inch reduction with placebo
Systolic blood pressure:
6.2 mm Hg reduction
1.1 mm Hg reduction with placebo
Supportive secondary end point: Change in6,8§:
Lipid profile:
Total cholesterol
3.3% reduction
0.1% increase with placebo
LDL
2.5% reduction
1.3% increase with placebo
HDL
5.2% increase
1.4% increase with placebo
Triglycerides
21.9% reduction
7.3% reduction with placebo
Diastolic blood pressure:
2.8 mm Hg reduction
0.4 mm Hg reduction with placebo
A1c:
0.4% reduction
0.2% reduction with placebo
Safety end point: Change in6:
Heart rate:
3.5 bpm increase
0.7 bpm reduction with placebo
Wegovy® is not indicated to treat hypertension, type 2 diabetes, or dyslipidemia.
The supportive secondary efficacy end points were not included in the statistical testing hierarchy and, as such, the analyses were not adjusted for multiplicity.
§Least squares mean change from baseline.
A1c, glycated hemoglobin; bpm, beats per minute; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Study design6
STEP 1: A 68-week trial of 1,961 adults with obesity (BMI ≥30 kg/m2) or with overweight (BMI 27 kg/m2-29.9 kg/m2) and at least one weight-related comorbid condition, such as treated or untreated dyslipidemia or hypertension; patients with type 2 diabetes mellitus were excluded. Patients were randomized in a 2:1 ratio to either once-weekly Wegovy® 2.4 mg or placebo (with a 16-week dose escalation), both in conjunction with a reduced-calorie diet (~500 kcal/day deficit) and increased physical activity (recommended to a minimum of 150 min/week).
Co-primary end points6,8:
- Mean percentage change in body weight from baseline to week 68
- Percentage of patients achieving ≥5% weight loss from baseline to week 68
Relevant confirmatory secondary end points6,8:
- Percentage of patients achieving ≥10% weight loss from baseline to week 68
- Percentage of patients achieving ≥15% weight loss from baseline to week 68
- Change in waist circumference from baseline to week 68
- Change in systolic blood pressure from baseline to week 68
Relevant supportive secondary end points6,8:
- Percentage of patients achieving ≥20% weight loss from baseline to week 68
- Change in A1c from baseline to week 68
- Change in diastolic blood pressure from baseline to week 68
- Change in lipids‖ from baseline to week 68
‖LDL, HDL, triglycerides, and total cholesterol.
In adults with obesity or overweight with at least one weight-related comorbidity, along with diet and exercise,
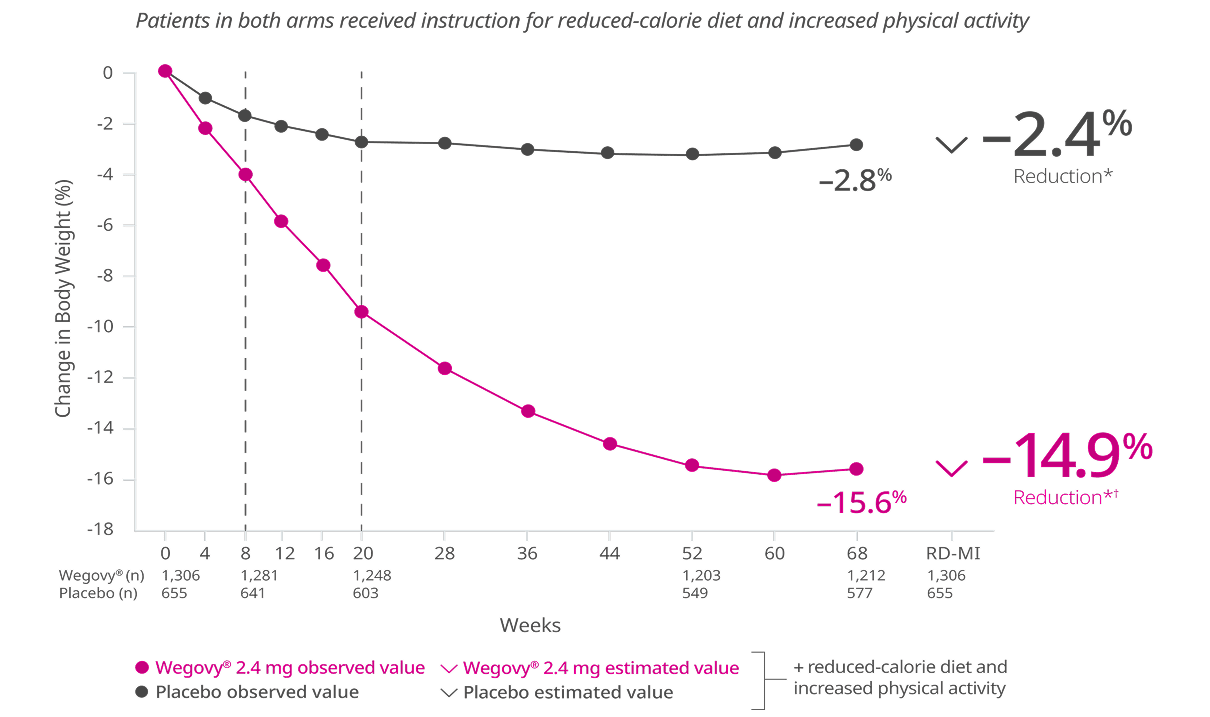
Once-weekly Wegovy® 2.4 mg delivered superior weight loss vs liraglutide 3 mg taken once daily subcutaneously6,9
Primary end point: Mean change in body weight (%) from week 0 to week 68

Missing data were imputed from retrieved subjects of the same randomized treatment arm (RD-MI).6
*p<0.001 (unadjusted 2-sided) for superiority.
†Difference from liraglutide 3 mg was -9.4%.
At 68 weeks, patients achieved9

STEP 8: A 68-week trial of 338 adults with obesity or with overweight and at least one weight-related comorbidity randomized in a 3:1:3:1 ratio to receive Wegovy® 2.4 mg or once-weekly matching placebo or liraglutide 3 mg or once-daily matching placebo. Discontinuation rate: Wegovy®=13.5%, liraglutide 3 mg=27.6%. Click to see full study design below.
In adults with obesity or overweight with at least one weight-related comorbidity, along with diet and exercise,
Significantly more patients achieved ≥10%, ≥15%, and ≥20% weight loss with Wegovy® vs liraglutide 3 mg6,9
Confirmatory secondary end points: Percent of participants achieving ≥10%, ≥15%, and ≥20% weight loss at week 68

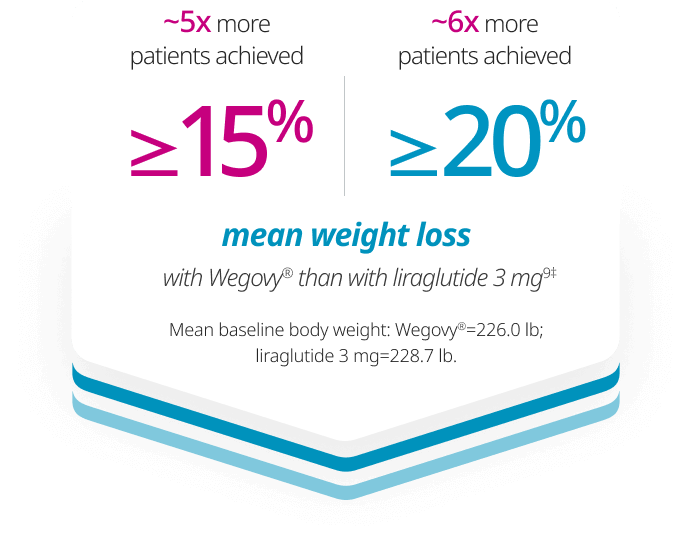
‡4.6x more patients achieved ≥15% weight loss and 6.4x more patients achieved ≥20% weight loss with Wegovy® than with liraglutide 3 mg at 68 weeks, respectively.
Weight loss in pounds (lb) calculated as 10%, 15%, and 20% of mean baseline body weight of Wegovy® and liraglutide 3 mg patients.
Observed data include only patients who had a body weight assessment at week 68 (117 of 126 for Wegovy® and 117 of 127 for liraglutide 3 mg) and do not include all randomized patients.9
In adults with obesity or overweight with at least one weight-related comorbidity, along with diet and exercise,
Patients taking Wegovy® experienced improvements in some cardiometabolic risk factors9
Supportive secondary end points: Changes in cardiometabolic risk factors from baseline at week 68
Supportive secondary end points: Change in9:
Lipid profile:
Total cholesterol
7.1% reduction
0.1% reduction with
liraglutide 3 mg
LDL
6.5% reduction
0.9% increase with liraglutide 3 mg
HDL
0.3% reduction
1.9% increase with liraglutide 3 mg
Triglycerides
20.7% reduction
11.0% reduction with liraglutide 3 mg
Waist circumference:
5.2-inch reduction
2.6-inch reduction with liraglutide 3 mg
Systolic blood pressure:
5.7 mm Hg reduction
2.9 mm Hg reduction with liraglutide 3 mg
Diastolic blood pressure:
5.0 mm Hg reduction
0.5 mm Hg reduction with liraglutide 3 mg
A1c:
0.2% reduction
0.1% reduction with liraglutide 3 mg
Safety end point: Change in9:
Heart rate:
5.4 bpm increase
4.3 bpm increase with
liraglutide 3 mg
1.2 bpm increase with pooled placebo
Wegovy® is not indicated to treat hypertension, type 2 diabetes, or dyslipidemia.
The supportive secondary efficacy end points were not included in the statistical testing hierarchy and, as such, the analyses were not adjusted for multiplicity.
Study design9
STEP 8: A 68-week, randomized, open-label trial that studied the efficacy and safety of Wegovy® 2.4 mg vs liraglutide 3 mg, both in conjunction with lifestyle intervention, in 338 adult patients with obesity (BMI ≥30 kg/m2) or overweight (BMI 27 kg/m2-29.9 kg/m2) and at least one weight-related comorbid condition (eg, hypertension, dyslipidemia, obstructive sleep apnea, cardiovascular disease), excluding diabetes. Patients were randomized in a 3:1:3:1 ratio to receive once-weekly Wegovy® 2.4 mg (16-week dose escalation; n=126) or once-weekly matching placebo, once-daily liraglutide 3 mg (4-week dose escalation; n=127), or once-daily matching placebo; active treatment groups were double-blinded against placebo.
Efficacy was evaluated for Wegovy® vs liraglutide 3 mg:
Primary end point9:
- Percent change from baseline in body weight at week 68
Confirmatory secondary end points9:
- Achievement of ≥10%, ≥15%, and ≥20% weight loss at week 68
Relevant supportive secondary end points9:
- Change in waist circumference from baseline to week 68
- Change in systolic and diastolic blood pressure from baseline to week 68
- Change in A1c from baseline to week 68
- Change in lipids§ from baseline to week 68
Trial limitations: Different dosing instructions for Wegovy® and liraglutide 3 mg may have contributed to differences in discontinuation rates and efficacy results. Randomization to active treatment was not masked due to dosing differences, but potential bias was mitigated by matched double-blind placebo controls. Missing data were handled through multiple imputation, which could potentially introduce bias, but number of participants with missing data was low due to high trial retention.9
§LDL, HDL, triglycerides, and total cholesterol.
A1c, glycated hemoglobin; BMI, body mass index; bpm, beats per minute; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
See Wegovy® and MACE risk reduction data
Get your patients started on Wegovy®
MACE, major adverse cardiovascular events.
Important Safety Information for Wegovy®
WARNING: RISK OF THYROID C-CELL TUMORS
- In rodents, semaglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether Wegovy® causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined
- Wegovy® is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of Wegovy® and inform them of symptoms of thyroid tumors (e.g. a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with Wegovy®
Contraindications
- Wegovy® is contraindicated in patients with a personal or family history of MTC or in patients with MEN 2, and in patients with a prior serious hypersensitivity reaction to semaglutide or to any of the excipients in Wegovy®. Serious hypersensitivity reactions, including anaphylaxis and angioedema have been reported with Wegovy®
Warnings and Precautions
- Risk of Thyroid C-Cell Tumors: Patients should be further evaluated if serum calcitonin is measured and found to be elevated or thyroid nodules are noted on physical examination or neck imaging
- Acute Pancreatitis: Acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with GLP-1 receptor agonists, including Wegovy®. Observe patients carefully for signs and symptoms of acute pancreatitis (including persistent severe abdominal pain, sometimes radiating to the back, and which may or may not be accompanied by vomiting). If acute pancreatitis is suspected, discontinue Wegovy® and initiate appropriate management
- Acute Gallbladder Disease: Treatment with Wegovy® is associated with an increased occurrence of cholelithiasis and cholecystitis. The incidence of cholelithiasis and cholecystitis was higher in Wegovy® pediatric patients aged 12 years and older than in Wegovy® adults. In clinical trials in adult patients, cholelithiasis was reported by 1.6% of Wegovy® patients and 0.7% of placebo patients. Cholecystitis was reported by 0.6% of Wegovy® patients and 0.2% of placebo patients. In a clinical trial in pediatric patients aged 12 years and older, cholelithiasis was reported by 3.8% of Wegovy® patients and 0% placebo patients. Cholecystitis was reported by 0.8% of Wegovy® pediatric patients and 0% placebo patients. Substantial or rapid weight loss can increase the risk of cholelithiasis; however, the incidence of acute gallbladder disease was greater in Wegovy® patients than in placebo patients, even after accounting for the degree of weight loss. If cholelithiasis is suspected, gallbladder studies and appropriate clinical follow-up are indicated
- Hypoglycemia: Wegovy® lowers blood glucose and can cause hypoglycemia. In a trial of adult patients with type 2 diabetes, hypoglycemia was reported in 6.2% of Wegovy® patients versus 2.5% of placebo patients. Patients with diabetes taking Wegovy® with an insulin or insulin secretagogue (e.g. sulfonylurea) may have an increased risk of hypoglycemia, including severe hypoglycemia. The use of Wegovy® in patients with type 1 diabetes or in combination with insulin has not been evaluated. Inform patients of the risk of hypoglycemia and educate them on the signs and symptoms. Monitor blood glucose in patients with diabetes
- Acute Kidney Injury: There have been postmarketing reports of acute kidney injury and worsening of chronic renal failure, which in some cases required hemodialysis, in patients treated with semaglutide. Patients with renal impairment may be at a greater risk of acute kidney injury, but some events have been reported in patients without known underlying renal disease. A majority of the events occurred in patients who experienced nausea, vomiting, or diarrhea, leading to volume depletion. Monitor renal function when initiating or escalating doses of Wegovy® in patients reporting severe adverse gastrointestinal reactions and in patients with renal impairment reporting any adverse reactions that could lead to volume depletion
- Severe Gastrointestinal Adverse Reactions: Use of Wegovy® has been associated with gastrointestinal adverse reactions, sometimes severe. In clinical trials, severe gastrointestinal adverse reactions were reported more frequently among patients receiving Wegovy® (4.1%) than placebo (0.9%). Wegovy® is not recommended in patients with severe gastroparesis
- Hypersensitivity Reactions: Serious hypersensitivity reactions (e.g., anaphylaxis, angioedema) have been reported with Wegovy®. If hypersensitivity reactions occur, discontinue use of Wegovy®, treat promptly per standard of care, and monitor until signs and symptoms resolve. Use caution in a patient with a history of anaphylaxis or angioedema with another GLP-1 receptor agonist
- Diabetic Retinopathy Complications in Patients with Type 2 Diabetes: In a trial of adult patients with type 2 diabetes, diabetic retinopathy was reported by 4.0% of Wegovy® patients and 2.7% of placebo patients. Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy. Patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy
- Heart Rate Increase: Mean increases in resting heart rate of 1 to 4 beats per minute (bpm) were observed in Wegovy® adult patients compared to placebo in clinical trials. More Wegovy® adult patients compared with placebo had maximum changes from baseline of 10 to 19 bpm (41% versus 34%) and 20 bpm or more (26% versus 16%). In a clinical trial in pediatric patients aged 12 years and older with normal baseline heart rate, more patients treated with Wegovy® compared to placebo had maximum changes in heart rate of 20 bpm or more (54% versus 39%). Monitor heart rate at regular intervals and instruct patients to report palpitations or feelings of a racing heartbeat while at rest. If patients experience a sustained increase in resting heart rate, discontinue Wegovy®
- Suicidal Behavior and Ideation: Suicidal behavior and ideation have been reported in clinical trials with other weight management products. Monitor patients for depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior. Discontinue Wegovy® in patients who experience suicidal thoughts or behaviors and avoid in patients with a history of suicidal attempts or active suicidal ideation
- Pulmonary Aspiration During General Anesthesia or Deep Sedation: Wegovy® delays gastric emptying. There have been rare postmarketing reports of pulmonary aspiration in patients receiving GLP-1 receptor agonists undergoing elective surgeries or procedures requiring general anesthesia or deep sedation who had residual gastric contents despite reported adherence to preoperative fasting recommendations. Instruct patients to inform healthcare providers prior to any planned surgeries or procedures if they are taking Wegovy®
Adverse Reactions
- Most common adverse reactions (incidence ≥5%) are: nausea, diarrhea, vomiting, constipation, abdominal pain, headache, fatigue, dyspepsia, dizziness, abdominal distention, eructation, hypoglycemia in patients with type 2 diabetes, flatulence, gastroenteritis, gastroesophageal reflux disease, and nasopharyngitis
Drug Interactions
- The addition of Wegovy® in patients treated with insulin has not been evaluated. When initiating Wegovy®, consider reducing the dose of concomitantly administered insulin secretagogues (such as sulfonylureas) or insulin to reduce the risk of hypoglycemia
- Wegovy® causes a delay of gastric emptying and has the potential to impact the absorption of concomitantly administered oral medications. Monitor the effects of oral medications concomitantly administered with Wegovy®
Use in Specific Populations
- Pregnancy: May cause fetal harm. When pregnancy is recognized, discontinue Wegovy®. Discontinue Wegovy® in patients at least 2 months before a planned pregnancy
- Pediatric: Adverse reactions with Wegovy® in pediatric patients aged 12 years and older were similar to those reported in adults. Pediatric patients ≥12 years of age treated with Wegovy® had greater incidences of cholelithiasis, cholecystitis, hypotension, rash, and urticaria compared to adults treated with Wegovy®. There are insufficient data in pediatric patients with type 2 diabetes treated with Wegovy® for obesity to determine if there is an increased risk of hypoglycemia with Wegovy® treatment similar to that reported in adults
- Geriatric: In the cardiovascular outcomes trial, patients aged 75 years and older reported more hip and pelvis fractures on Wegovy® than placebo. Patients aged 75 years and older (Wegovy® and placebo) reported more serious adverse reactions overall compared to younger adult patients
Please click here for Wegovy® Prescribing Information, including Boxed Warning.
Indications and Usage
Wegovy® (semaglutide) injection 2.4 mg is indicated in combination with a reduced calorie diet and increased physical activity:
- to reduce the risk of major adverse cardiovascular events (cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke) in adults with established cardiovascular disease and either obesity or overweight
- to reduce excess body weight and maintain weight reduction long term in adults and pediatric patients aged 12 years and older with obesity and adults with overweight in the presence of at least one weight-related comorbidity
Limitations of Use:
Wegovy® contains semaglutide. Coadministration with other semaglutide-containing products or with any GLP-1 receptor agonist is not recommended
Important Safety Information for Wegovy®
WARNING: RISK OF THYROID C-CELL TUMORS
- In rodents, semaglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether Wegovy® causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined
- Wegovy® is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of Wegovy® and inform them of symptoms of thyroid tumors (e.g. a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with Wegovy®
Important Safety Information for Wegovy®
WARNING: RISK OF THYROID C-CELL TUMORS
- In rodents, semaglutide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether Wegovy® causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined
- Wegovy® is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of Wegovy® and inform them of symptoms of thyroid tumors (e.g. a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with Wegovy®
Contraindications
- Wegovy® is contraindicated in patients with a personal or family history of MTC or in patients with MEN 2, and in patients with a prior serious hypersensitivity reaction to semaglutide or to any of the excipients in Wegovy®. Serious hypersensitivity reactions, including anaphylaxis and angioedema have been reported with Wegovy®
Warnings and Precautions
- Risk of Thyroid C-Cell Tumors: Patients should be further evaluated if serum calcitonin is measured and found to be elevated or thyroid nodules are noted on physical examination or neck imaging
- Acute Pancreatitis: Acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with GLP-1 receptor agonists, including Wegovy®. Observe patients carefully for signs and symptoms of acute pancreatitis (including persistent severe abdominal pain, sometimes radiating to the back, and which may or may not be accompanied by vomiting). If acute pancreatitis is suspected, discontinue Wegovy® and initiate appropriate management
- Acute Gallbladder Disease: Treatment with Wegovy® is associated with an increased occurrence of cholelithiasis and cholecystitis. The incidence of cholelithiasis and cholecystitis was higher in Wegovy® pediatric patients aged 12 years and older than in Wegovy® adults. In clinical trials in adult patients, cholelithiasis was reported by 1.6% of Wegovy® patients and 0.7% of placebo patients. Cholecystitis was reported by 0.6% of Wegovy® patients and 0.2% of placebo patients. In a clinical trial in pediatric patients aged 12 years and older, cholelithiasis was reported by 3.8% of Wegovy® patients and 0% placebo patients. Cholecystitis was reported by 0.8% of Wegovy® pediatric patients and 0% placebo patients. Substantial or rapid weight loss can increase the risk of cholelithiasis; however, the incidence of acute gallbladder disease was greater in Wegovy® patients than in placebo patients, even after accounting for the degree of weight loss. If cholelithiasis is suspected, gallbladder studies and appropriate clinical follow-up are indicated
- Hypoglycemia: Wegovy® lowers blood glucose and can cause hypoglycemia. In a trial of adult patients with type 2 diabetes, hypoglycemia was reported in 6.2% of Wegovy® patients versus 2.5% of placebo patients. Patients with diabetes taking Wegovy® with an insulin or insulin secretagogue (e.g. sulfonylurea) may have an increased risk of hypoglycemia, including severe hypoglycemia. The use of Wegovy® in patients with type 1 diabetes or in combination with insulin has not been evaluated. Inform patients of the risk of hypoglycemia and educate them on the signs and symptoms. Monitor blood glucose in patients with diabetes
- Acute Kidney Injury: There have been postmarketing reports of acute kidney injury and worsening of chronic renal failure, which in some cases required hemodialysis, in patients treated with semaglutide. Patients with renal impairment may be at a greater risk of acute kidney injury, but some events have been reported in patients without known underlying renal disease. A majority of the events occurred in patients who experienced nausea, vomiting, or diarrhea, leading to volume depletion. Monitor renal function when initiating or escalating doses of Wegovy® in patients reporting severe adverse gastrointestinal reactions and in patients with renal impairment reporting any adverse reactions that could lead to volume depletion
- Severe Gastrointestinal Adverse Reactions: Use of Wegovy® has been associated with gastrointestinal adverse reactions, sometimes severe. In clinical trials, severe gastrointestinal adverse reactions were reported more frequently among patients receiving Wegovy® (4.1%) than placebo (0.9%). Wegovy® is not recommended in patients with severe gastroparesis
- Hypersensitivity Reactions: Serious hypersensitivity reactions (e.g., anaphylaxis, angioedema) have been reported with Wegovy®. If hypersensitivity reactions occur, discontinue use of Wegovy®, treat promptly per standard of care, and monitor until signs and symptoms resolve. Use caution in a patient with a history of anaphylaxis or angioedema with another GLP-1 receptor agonist
- Diabetic Retinopathy Complications in Patients with Type 2 Diabetes: In a trial of adult patients with type 2 diabetes, diabetic retinopathy was reported by 4.0% of Wegovy® patients and 2.7% of placebo patients. Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy. Patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy
- Heart Rate Increase: Mean increases in resting heart rate of 1 to 4 beats per minute (bpm) were observed in Wegovy® adult patients compared to placebo in clinical trials. More Wegovy® adult patients compared with placebo had maximum changes from baseline of 10 to 19 bpm (41% versus 34%) and 20 bpm or more (26% versus 16%). In a clinical trial in pediatric patients aged 12 years and older with normal baseline heart rate, more patients treated with Wegovy® compared to placebo had maximum changes in heart rate of 20 bpm or more (54% versus 39%). Monitor heart rate at regular intervals and instruct patients to report palpitations or feelings of a racing heartbeat while at rest. If patients experience a sustained increase in resting heart rate, discontinue Wegovy®
- Suicidal Behavior and Ideation: Suicidal behavior and ideation have been reported in clinical trials with other weight management products. Monitor patients for depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior. Discontinue Wegovy® in patients who experience suicidal thoughts or behaviors and avoid in patients with a history of suicidal attempts or active suicidal ideation
- Pulmonary Aspiration During General Anesthesia or Deep Sedation: Wegovy® delays gastric emptying. There have been rare postmarketing reports of pulmonary aspiration in patients receiving GLP-1 receptor agonists undergoing elective surgeries or procedures requiring general anesthesia or deep sedation who had residual gastric contents despite reported adherence to preoperative fasting recommendations. Instruct patients to inform healthcare providers prior to any planned surgeries or procedures if they are taking Wegovy®
Adverse Reactions
- Most common adverse reactions (incidence ≥5%) are: nausea, diarrhea, vomiting, constipation, abdominal pain, headache, fatigue, dyspepsia, dizziness, abdominal distention, eructation, hypoglycemia in patients with type 2 diabetes, flatulence, gastroenteritis, gastroesophageal reflux disease, and nasopharyngitis
Drug Interactions
- The addition of Wegovy® in patients treated with insulin has not been evaluated. When initiating Wegovy®, consider reducing the dose of concomitantly administered insulin secretagogues (such as sulfonylureas) or insulin to reduce the risk of hypoglycemia
- Wegovy® causes a delay of gastric emptying and has the potential to impact the absorption of concomitantly administered oral medications. Monitor the effects of oral medications concomitantly administered with Wegovy®
Use in Specific Populations
- Pregnancy: May cause fetal harm. When pregnancy is recognized, discontinue Wegovy®. Discontinue Wegovy® in patients at least 2 months before a planned pregnancy
- Pediatric: Adverse reactions with Wegovy® in pediatric patients aged 12 years and older were similar to those reported in adults. Pediatric patients ≥12 years of age treated with Wegovy® had greater incidences of cholelithiasis, cholecystitis, hypotension, rash, and urticaria compared to adults treated with Wegovy®. There are insufficient data in pediatric patients with type 2 diabetes treated with Wegovy® for obesity to determine if there is an increased risk of hypoglycemia with Wegovy® treatment similar to that reported in adults
- Geriatric: In the cardiovascular outcomes trial, patients aged 75 years and older reported more hip and pelvis fractures on Wegovy® than placebo. Patients aged 75 years and older (Wegovy® and placebo) reported more serious adverse reactions overall compared to younger adult patients
Please click here for Wegovy® Prescribing Information, including Boxed Warning.
Indications and Usage
Wegovy® (semaglutide) injection 2.4 mg is indicated in combination with a reduced calorie diet and increased physical activity:
- to reduce the risk of major adverse cardiovascular events (cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke) in adults with established cardiovascular disease and either obesity or overweight
- to reduce excess body weight and maintain weight reduction long term in adults and pediatric patients aged 12 years and older with obesity and adults with overweight in the presence of at least one weight-related comorbidity
Limitations of Use:
Wegovy® contains semaglutide. Coadministration with other semaglutide-containing products or with any GLP-1 receptor agonist is not recommended
References
1. Garvey WT, Batterham RL, Bhatta M, et al. Two-year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial. Nat Med. 2022;28(10):2083-2091.
2. Zabor EC, Kaizer AM, Hobbs BP. Randomized controlled trials. Chest. 2020;158(1S):S79-S87.
3. Sheldrick RC. Randomized trials vs real-world evidence: how can both inform decision-making? JAMA. 2023;329(16):1352-1353.
4. Dang A. Real-world evidence: a primer. Pharmeceut Med. 2023;37(1):25-36.
5. Data on file. Novo Nordisk Inc.; Plainsboro, NJ.
6. Wegovy® [package insert]. Plainsboro, NJ: Novo Nordisk Inc.
7. Rubino D, Abrahamsson N, Davies M, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA. 2021;325(14):1414-1425.
8. Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002.
9. Rubino DM, Greenway FL, Khalid U, et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: the STEP 8 randomized clinical trial. JAMA. 2022;327(2):138-150.